Assessment of radiofrequency ablation technique in development of aortic valve stenosis in rabbits
by F Zeinali1, K. E. Bakkelund1, Ø Hauso2, J. P. Loennechen3,4, H. L. Waldum1,2
1Department of Cancer Research and Molecular Medicine, Faculty of
Medicine, NTNU, Trondheim, Norway
2Department of Gastroenterology and Liver Diseases, St. Olavs Hospital
HF, Trondheim University Hospital, Trondheim,
Norway
3Department of Circulation and Medical Imaging, Faculty of Medicine,
NTNU, Trondheim, Norway
4Department of Cardiology, St. Olavs Hospital, Trondheim, Norway
 Correspondence: Dr Fatemeh Zeinali
Correspondence: Dr Fatemeh Zeinali
Department of Cancer Research and Molecular Medicine, NTNU,
Olav Kyrresgate 3, NO-7489, Trondheim, Norway.
Mobile +47 72829958
Tel +47 72829950
Email fatemeh.zeinali@ntnu.no
Summary
Purpose: To develop a minimally invasive and
reproducible model of aortic stenosis in rabbits
using radiofrequency ablation technique (RFA).
Material and methods: Eleven rabbits were studied. A
radiofrequency ablation catheter was
introduced via the femoral artery and advanced to the aortic valve
area under fl uoroscopic control.
In three rabbits radiofrequency energies, at 5 W, 10 W and 15 W
respectively, were applied
thrice for 90 sec. In eight rabbits, energy of 15 W was applied for
the same time periods. Th e
velocity of the blood through the aortic valve was determined by color
Doppler ultrasound
immediately before and aft er ablation and aft er six weeks. Aft er
six weeks the rabbits were sacrifi
ced and the aortic valve was examined macroscopically.
Results: Peak systolic velocity (PSV) was similar at
the time of ablation and aft er six weeks in
eight of the nine surviving rabbits, and had increased from 1.1 to
1.75 m/s in one rabbit. Two
rabbits developed aortic insuffi ciencies visualized by color Doppler.
No macroscopic changes
were seen at the aortic valve area 6 weeks aft er ablation.
Conclusion: In the current study we did not succeed
in inducing aortic valve damage/fi brosis
using diff erent RFA energies. Inadequate RFA
Introduction
Blood serotonin in patients with serotonin-producing neuroendocrine
tumors (carcinoid tumors) is considered to cause valvular heart
disease (Robiolio et al. 1995) . During the last decade,
several drugs acting on serotonin receptors have been recognized to
induce cardiac valvulopathy. Appetite-suppressants such as
fenfluramine were the first drugs described to cause valvular heart
disease (Rothman et al., 1999). Serotonin induces heart valve
disease in rats after long-term serotonin administration (Gustafsson et al., 2005) and terguride, which has serotonin antagonist activity, decreases
heart disease in rats exposed to long-term hyperserotoninemia (Hauso et al., 2007) . Despite this evidence, it is unclear whether serotonin may also
play a role in the development of common valvular heart disease. The
fact that people with normal, but bicuspid aortic valves, develop
progressive valvular disease (Ward, 2000) indicates that flow
disturbances may provoke valvular disease. Hemodynamic disturbances
with accelerated and turbulent flow probably activate platelets and
may cause release of serotonin from dense granules (Brandt et al., 1992). Our hypothesis is that platelet-derived serotonin may be a crucial
factor in the development of valvular heart disease. Therefore a
suitable animal model of aortic stenosis is required to investigate
disease mechanisms and potential therapies. Most of the experimental
animal models for this disease have been based on the development of
atherosclerotic plaques which have the disadvantage of being
time-consuming (Johnson & Jackson, 2001; Riedmuller et al., 2010)
and also involve non-mechanical changes. Mechanical techniques to
produce aortic stenosis, including ligation of aortic leaflets (Copeland et al., 1974) and ascending aorta banding (Taylor & Whamond, 1975),
have been used. However, these methods often result in aortic rupture
in adult animals or valvular insufficiency. Presently, there is no
good method to induce aortic valvular stenosis in small animals.
The aim of the current study was to develop a minimally and
reproducible model of aortic stenosis in rabbits by using the
radiofrequency ablation technique (RFA).
RFA has been established as one of the treatment options for cardiac
tachy-arrhythmias (Nath et al. 1994). It has also been used
for treatment of varicose veins (Marsh et al., 2010).
Recently a new rabbit model of arterial luminal stenosis was reported,
by endovascular application of radiofrequency (RF) energy in the
aorta below the level of the renal arteries (Lazoura et al., 2011). In the present study, we wanted to establish a new experimental
model based on a similar technique using RFA on rabbit aortic valves
to provoke valvular damage/fibrosis. The intention was to induce blood
flow disturbances which would possibly activate platelets and in the
end lead to the development of progressive valvular aortic stenosis.
Materials and methods
The National Animal Welfare Committee approved the study. Eleven New
Zealand White male rabbits (Harlan Laboratories, UK), 14-16 weeks old,
weighing 3-4 kg were housed individually in cages. Concerning their
health and microbiological status, positive results for
Eimeria spp and Passalurus ambiguus have been found
in the past, but not in the last 18 months.
Room temperature was 24±1°C with a 12-hour light/dark cycle. A
commercial pellet diet (Scanbur, Karlslunde, DK), dried grass and
water were supplied ad libitum. Before all procedures, each
animal was premedicated with Hypnorm (Vetapharma Ltd, Leeds UK) 0.15
ml/kg sc.
RF generator and catheter
We used a Medtronic Atakr II radiofrequency generator and a Medtronic
Marinr 5 Fr, 35 mm reach radiofrequency ablation catheter with a 4 mm
platinum tip. The high frequency current passed between the electrode
and an ablation pad applied to the shaved back of the rabbits.
Rabbit model development
We aimed to induce significant valvular aortic stenosis using the
minimum amount of energy, without vessel rupture or any other
complications.
After premedication, each animal was anesthetized with a high dose of
isofluran (5%, 3 to 5 minutes) until deep anesthesia, continuing with
1.5 to 2% isofluran and oxygen / nitrous oxide in a ratio of 40/60
during the procedure. The chest was carefully shaved. Using a GE
Vingmed Vivid 7 ultrasound scanner and a GE Vingmed M4s 1.5-4.0 MHz
phase arrayed ultrasound probe the peak velocity of the aortic valve
blood flow was measured as the mean of 5 cycles of continuous Doppler
recordings. The rabbit was placed in the supine position. Following
shaving, a sterile drape was applied to right ventral femoral area and
a ~2-cm vertical incision was performed. The right femoral artery was
dissected free and ligated at its distal end, followed by dripping
papaverin upon the vessel to reduce spasm tendency. The right femoral
artery was then cut open to place a 4 Fr introducer sheath (Introducer
II, Radiofocus) for dilation, followed by the insertion of a 5 Fr
heparinized (5000 E heparin/100 ml water) introducer sheath. The 5 Fr
heparinized RF catheter was then advanced to the aortic valve, away
from the septum to reduce the risk of AV block. The procedure was
performed under radiological control by X-ray and the aortic catheter
position was verified by echocardiography.
In 3 rabbits temperature controlled (55 degrees C)
radiofrequency energy was applied at 5 W, 10 W and 15 W respectively 3
times for 90 sec. In the next 8 rabbits a similar procedure using 15 W
was applied. Following completion of the procedure, the sheath was
removed, the common femoral artery was ligated and the muscle layers
and skin were closed with 3.0 Vicryl resorbable sutures. Color Doppler
ultrasound was also performed after the procedure, to measure the
blood flow velocity at the aortic valve. Finally Temgesic (Reckitt
Benckiser, Berkshire, UK, 0.02 mg/kg) was administered as an
analgesca. Animals showing any discomfort suggesting pain were given
another dose of Temgesic 3-4 hours after the procedure. The surviving
rabbits were examined by color Doppler ultrasound six weeks after the
procedure and sacrificed to look for macroscopic changes.
The rabbits were euthanized with phenobarbital 30 mg/kg i.v at the
termination of study (or directly after a failed procedure) or earlier
if the rabbits suffered from breathlessness, signs of pain or
developed progressive weight loss. The rabbits were monitored several
times daily by the personnel of the animal department.
Echocardiography and Macroscopic examination
The rabbits were examined by echocardiography before, during and after
the procedure as well as during follow up. Two-dimensional color-flow
Doppler in the parasternal long -and short axis views was used to
visualize aortic flow and regurgitation, and the peak systolic
velocities (PSV) through the aortic valve were measured.
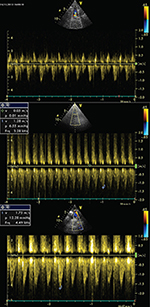
Continuous-wave Doppler recordings of rabbit No.4 are shown in Figure
1.
Sodium pentobarbital, 30 mg/kg i.v was administered intravenously six
weeks after the procedure in all rabbits. We then performed a median
sternotomy, carefully removed adhering tissue around the myocardium,
and excised the hearts with the aortic root (Figure 2).
Results
Nine rabbits survived the experimental procedure. Two rabbits died
instantly after the procedure because of complete atrioventricular
block. Information about the animals and Doppler flow measurements for
each application of RF energy of aortic valve is detailed in Table 1.
Rabbit No.3 developed cardiac arrest due to ventricular fibrillation
during the second 90 sec ablation period (power of 15 W). After
resuscitation, by use of heart compressions, the rabbit lived for one
more week. The cause of death was probably heart failure caused by
large amounts of cardiac effusion (found on autopsy) possibly
secondary to a myocardial rupture. In rabbit No.6 we planned to apply
RFA power of 20 W, but at 15 W maximum temperature was achieved and
the system automatically stopped delivering RFA energy.
Aortic insufficiency was found 6 weeks after ablation with 15 W in two
rabbits. PSV through the aortic valve at six weeks after ablation was
similar in all animals except in one rabbit (No.4) with increased PSV
and which also had aortic insufficiency (Table 1). No changes were
identified by macroscopic examination.
Table 1. Radiofrequency ablation (RFA) of the aortic valves (power, time and numbers of applications) and peak systolic velocity determined by color Doppler ultrasonography before, immediately after RFA and 6 weeks after RFA.
| Rabbit | Power | Time | 1RF applications | 2PSV before RF | PSV after RF | Follow-up PSV |
| No | (Watt) | (s) | (time) | (m/s) | (m/s) | (m/s) |
| 1 | 5 | 90 | 3 | 1.1 | 1.1 | 1.0 |
| 2 | 10 | 90 | 3 | 1.2 | 1.05 | 0.9 |
| 3 | 15 | 90.73 | 3 | 1.0 | 0.95 | - |
| 4* | 15 | 90 | 3 | 1.1 | 1.28 | 1.75 |
| 5* | 15 | 90 | 3 | 0.9 | 1.0 | 1.0 |
| 6 | 15 | 90 | 3 | 1.18 | 1.15 | 1.0 |
| 7 | 15 | 90 | 3 | 1.1 | 1.15 | 1.2 |
| 8 | 15 | 90 | 3 | 1.1 | 1.1 | 1.0 |
| 9 | 15 | 90 | 3 | 0.95 | 0.95 | 1.0 |
1RF: Radiofrequency, 2PSV: Peak systolic velocity, *Aortic insufficiency
Discussion
In the current study, we aimed to develop a minimally invasive and
reproducible model of progressive aortic stenosis in rabbits using
RFA. We did not succeed in inducing aortic valve stenosis using
different RFA energies. In one rabbit there was increased blood flow
velocity through the aortic valve which, however, most probably was
secondary to a large aortic insufficiency. We may have applied too
little energy to induce valvular changes. The RFA procedure, however,
induced ventricular fibrillation in one rabbit and two rabbits died
during the procedure due to complete heart block. Afurther increase in
RFA energy levels seemed futile. Furthermore, application of high
levels of energy may result in vessel rupture (Zacharoulis, 2011). We did only eleven examinations since we felt that we could not
proceed further both from an economical as well as an ethical point of
view. However, we cannot exclude the possibility that including more
animals may have given a different result.
Lazoura et al. (2011) developed a new rabbit model
of aortic luminal stenosis between the origins of the renal arteries
and the aortic bifurcation, based on endovascular RFA (Lazoura et al., 2011). The optimal RFA power to induce significant stenosis in their
study was 24-36 W for 1.5 min which was higher than we applied.
Another possible reason for our failure to induce aortic valve
stenosis may be incorrect placement of the RFA energy. Localization of
the aortic valve area is difficult, and in another study an
intracardiac catheter was used for accurate catheter placement (Doi et al., 2003). This technique was used in a canine model and may be difficult to
apply in small animals like rabbits.
Therefore inadequate RF power and inappropriate guidance to place RF
catheter could be the limitations of our study investigating a less
invasive method to induce aortic valve stenosis in a rabbit model.
Nevertheless, it may be possible to further explore and develop RFA
into a method to initiate progressive aortic valvular stenosis. Such a
method is required to develop and test new drugs with the ability to
stop progression of valvular disease.
Competing interests
The authors declare that they have no competing interests. The authors alone are responsible of the content and writing of the paper.
References
-
Copeland JG, BJ Maron, NL Luka, VJ Ferrans & LL Michaelis: Experimental production of aortic valvular stenosis. Short-term and long-term studies in dogs. J. Thorac. Cardiov. Sur. 1974, 67(3), 371-379.
-
Gustafsson BI, K Tommeras, I Nordrum, JP Loennechen, A Brunsvik, E Solligard, R Fossmark, I Bakke, U Syversen & H Waldum. Long-term serotonin administration induces heart valve disease in rats. Circulation 2005, 111(12), 1517-1522.
-
Hauso, O, BI Gustafsson, JP Loennechen, AK. Stunes, I Nordrum & HL Waldum: Long-term serotonin effects in the rat are prevented by terguride. Regul. Peptides 2007, 143(1-3), 39-46.
-
Johnson, JL & CL Jackson: Atherosclerotic plaque rupture in the apolipoprotein E knockout mouse. Atherosclerosis 2001, 154(2), 399-406.
-
Lazoura, O, D Zacharoulis, T Kanavou, C Rountas, M Katsimboulas, G Tzovaras & N Habib: A novel experimental animal model of arterial stenosis based on endovascular radiofrequency energy application. J. Invest. Surg. 2011, 24(3), 123-128.
-
Marsh P, BA Price, JM Holdstock & MS Whiteley: One-year outcomes of radiofrequency ablation of incompetent perforator veins using the radiofrequency stylet device. Phlebology 2010, 25(2), 79-84.
-
Nath, S, JP DiMarco & DE Haines: Basic aspects of radiofrequency catheter ablation. J. Cardiovasc. Electr. 1994, 5(10), 863-876.
-
Riedmuller K, S Metz, GA Bonaterra, O Kelber, D Weiser, J Metz & R Kinscherf: Cholesterol diet and effect of long-term withdrawal on plaque development and composition in the thoracic aorta of New Zealand White rabbits. Atherosclerosis 2010, 210(2), 407-413.
-
Robiolio PA, VH Rigolin, JS Wilson, JK Harrison, LL Sanders, TM Bashore & JM Feldman: Carcinoid heart disease. Correlation of high serotonin levels with valvular abnormalities detected by cardiac catheterization and echocardiography. Circulation 1995, 92(4), 790-795.
-
Rothman RB, MA Ayestas, CM Dersch & MH Baumann: Aminorex, fenfluramine, and chlorphentermine are serotonin transporter substrates. Implications for primary pulmonary hypertension. Circulation 1999, 100, 869-875.
-
Taylor DE & JS Whamond: A method of producing graded stenosis of the aortic and mitral valves in sheep for fluid dynamic studies. J. Physiol. 1975, 244(1), 16P-17P.
-
Ward C: Clinical significance of the bicuspid aortic valve. Heart 2000, 83(1), 81-85.
-
Zacharoulis D, O Lazoura, C Rountas, M Katsimboulas, G Mantzianas, G Tzovaras & N Habib: Experimental animal study of a novel radifrequency endovascular occlusion device. Am. J. Surg. 2011, 202(1), 103-109.