Surgical anatomy of the sural nerve for peripheral nerve reconstruction research in swine
by R Sasaki1, 2, H Matsumine2, 3, Y Watanabe2, 4 M Yamato2,, T Okano2 & Tomohiro Ando1
1Department of Oral and Maxillofacial Surgery, and Global Center of
Excellence (COE) program, Tokyo Women’s Medical University, School of
Medicine, Tokyo, Japan
2Institute of Advanced Biomedical Engineering and Science, Tokyo
Women’s Medical University, Tokyo, Japan
3Department of Plastic and Reconstructive Surgery, Tokyo Metropolitan
Police Hospital, Tokyo, Japan
4Department of Plastic and Reconstructive Surgery, Tokyo Metropolitan
Police Hospital, Tokyo, Japan
 Correspondence: Dr. Ryo Sasaki; Department of Oral and Maxillofacial Surgery, Tokyo
Women’s Medical University, School of Medicine, 8-1 Kawada-cho,
Shinjuku-ku, Tokyo 162-8666, Japan
Correspondence: Dr. Ryo Sasaki; Department of Oral and Maxillofacial Surgery, Tokyo
Women’s Medical University, School of Medicine, 8-1 Kawada-cho,
Shinjuku-ku, Tokyo 162-8666, Japan
Mobile +81-3-3353-8111
Tel +81-3-5269-2367
Email sasaki@oms.twmu.ac.jp
Summary
The use of peripheral nerves as donor nerves for peripheral nerve
regeneration studies, which can provide a long peripheral nerve with
intact physiological functions, for autologous nerve grafts was
unknown in swine. This study investigated the surgical anatomy of
sural nerves (nervus suralis) of cadavers and anesthetized miniature
pigs. A loose-S shape incision line was made from the border of the
biceps femoris muscle (musculus biceps femoris) to 2 cm above the calx
in the leg of anesthetized miniature pigs. The sural nerve was found
to branch from the sciatic nerve (nervus ischiadicus) under the biceps
femoris muscle and run along the small saphenous vein (vena saphena
parva). After being isolated and stimulated using a nerve stimulator,
the sural nerve innervated no muscles and tissues in the leg. The
sural nerve (14.5 ± 0.5 cm) was obtained from between the sciatic
nerve and peripheral branches in anesthetized miniature pigs.
Toluidine-blue staining of the obtained sural nerve indicated that the
numbers of myelinated fibers in the distal and proximal portions were
2271 ± 639 and 2639 ± 622, respectively. It is concluded that the
sural nerve of miniature pigs can be used for peripheral nerve
regeneration studies.
Introduction
Miniature pigs are a suitable experimental model in translational research, because their anatomical structure is similar to humans and, compared with rats and mice, their body size is closer to humans (Swindle et al., 1994; Vodicka et al., 2005; Wang et al., 2007; Sasaki et al., 2010). However, the use of peripheral nerves as donor nerves for peripheral nerve regeneration studies, which can provide a long peripheral nerve with intact physiological functions, for autologous nerve grafts was unknown in swine. The sural nerve (nervus suralis), a sensory nerve of the leg, is the most common peripheral nerve donor in human facial nerve reconstruction, because a long nerve can be easily obtained without functional defects (Doi et al., 1984; Kim & Seo, 2001). Therefore, in this study, the use of the sural nerve as a donor for autologous nerve grafts was investigated in miniature swine cadavers and anaesthetized miniature swine.
Materials and methods
The cadavers of 3 healthy 8-month-old NIBS miniature pigs (25.8-27.4 kg) were used for this study (Nisseiken, Ome, Japan). Prior to their use in this study, the three pigs were used in a cardiology or general surgery study and were euthanized at the end of that project by intravenous injection of KCl under deep sevoflurane-induced anesthesia. After the confirmation of death, the three cadavers were used for the present study. One cadaver was frozen at -15°C until used. Before the experiment, the frozen cadaver was kept overnight at room temperature.
For the anesthetized pig experiment, healthy 9- to 11-month-old male NIBS miniature pigs (Nisseiken) (26-30 kg) and a Clawn miniature pig (Japan Farm Clawn Institute, Kagoshima) were used. The pigs were first pre-medicated using a combined intramuscular injection of medetomidine (80 µg/kg), ketamine (5 mg/kg), and butorphanol (0.2 mg/kg) before being intubated and anesthetized by sevoflurane-induced anesthesia. The sural nerve was dissected. One week after surgery, functional defect, such as dragging the leg was evaluated.
Animal care and handling procedures were performed in accordance with the “Principles of Laboratory Animal Care” of the Tokyo Women’s Medical University Animal Experimentation Committee.
The distal and proximal portions (2 cm from the distal and proximal
end) of the obtained sural nerve were subsequently examined. Samples
were prefixed with 2% glutaraldehyde (distilled EM grade, EM Science,
Gibbstown, NJ, USA), 2% paraformaldehyde (Wako Pure Chemical, Osaka)
and 0.1 mol/L cacodylate buffer (Wako), and postfixed with 2% osmium
tetroxide (Wako) and 0.1 mol/L cacodylate buffer (Wako) before being
embedded in Quetol-812 resin (Nisshin EM, Tokyo, Japan) at 60°C for 2
days. The embedded specimens were cut into approximately 1.5 mm
sections with a glass knife and heat-stained with 0.5% toluidine blue.
The numbers of myelinated fibers in the distal and proximal portion of
the sural nerve specimens were manually counted on photographs taken
with a conventional microscope.
Results
Sural nerve dissection in miniature pig cadavers
The sural nerve was found to branch from the sciatic nerve under the
biceps femoris muscle (musculus biceps femoris) and to run along the
small saphenous vein (vena saphena parva). The sural nerve branched
into approximately 3 branches at the calx. The mean length of the
obtained sural nerve between the sciatic nerve and peripheral branches
in the miniature pig cadavers was 12.33 ± 1.2 cm (range: 10 to 14 cm,
n = 3, mean ± S.E.M.).
Sural nerve dissection in anesthetized miniature pigs
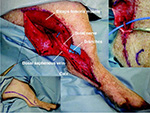
Based on the anatomy of the cadaver sural nerves, a loose-S shape
incision line was performed from the border of the biceps femoris
muscle to 2 cm above the calx in the leg of anesthetized miniature
pigs (Fig. 1, left below). The sural nerve in the pigs branched from
the sciatic nerve under the biceps femoris muscle and ran along the
small saphenous vein (Fig. 1, right above). The sural nerve branched
into approximately 3 branches at the calx (Fig. 1, central). The sural
nerve was isolated and then stimulated using a nerve stimulator
(Vari-stim® III, Medtronic, Jacksonville, FL). The nerve stimulation
results indicated that the sciatic nerve (nervus ischiadicus)
innervated muscles and tissues in the leg. However, the sural nerve
innervated no muscles and tissues. The sural nerve (14.5 ± 0.5 cm,
range: 13.5 to 15 cm, n = 3, mean ± S.E.M.) was obtained between the
sciatic nerve branch and peripheral branches in the anesthetized
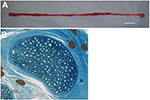
miniature pigs (Fig. 2-A). One week after surgery, no functional
defect, such as dragging the leg was observed. Moreover, no plantar
decubitus ulcer was observed at 3 months after surgery. Toluidine-blue
staining of the distal and proximal portions of the obtained sural
nerve indicated 5.33 ± 2.1 and 5.00 ± 1.5 distal and proximal stumps,
respectively. There were 2271 ± 639.5 and 2639 ± 622.7 myelinated
fibers in the distal and proximal portions, respectively (Fig. 2-B).
Discussion
Miniature swine are used as an experimental or pre-clinical model in maxillofacial surgery, including mandibular reconstruction (Terheyden et al., 1999) and distraction osteogenesis (Goldwaser et al., 2013), dental implantology (Eom et al., 2012), bisphosphonate-related jaw osteonecrosis (Li et al., 2013) and face transplantation (Kuo et al., 2012) because the anatomical structure of the maxillofacial region is similar to humans and, compared with rats and mice, their facial size is closer to humans (Swindle et al., 1994; Vodicka et al., 2005; Wang et al., 2007; Sasaki et al., 2010; Sasaki et al., 2014a; Sasaki et al., 2015). Miniature pigs are also suitable for surgical training and teaching for newly innovated transplantation methods (Swindle et al., 1994; Swindle and Smith, 2013). Although facial nerve regeneration studies using a tissue-engineered nerve guide are reported in rats (Sasaki et al., 2008; Sasaki et al., 2011; Matsumine et al., 2012; Sasaki et al., 2014b), no facial nerve regeneration study using miniature swine has been reported. Validation of an animal model more closely related to humans is needed in translational research (Vodicka et al., 2005). Therefore, the surgical anatomy of the face of miniature swine, including the buccal branch and marginal mandibular branch of the facial nerve, was investigated for facial nerve regeneration research in our previous study (Sasaki et al., 2010). Miniature pigs are a suitable experimental model for facial nerve regeneration surgery (Sasaki et al., 2010; Sasaki et al., 2014a). However the use of peripheral nerves as donor nerves, which can provide a long peripheral nerve without functional defects, for autologous nerve grafts as the control against novel tissue-engineered nerve guide was unknown in miniature pigs. The human sural nerve is the most common donor peripheral nerve in facial nerve reconstruction, because a long nerve can be easily obtained with intact physiological functions (Doi et al., 1984; Kim & Seo, 2001). Therefore, this study investigated use of the sural nerve as a donor for autologous nerve grafts in miniature swine. Interestingly, the sural nerve in pigs was found to be similar to the human sural nerve in that it is located parallel to the small saphenous vein (Doi et al., 1984; Kim & Seo, 2001). Although a maximum of 40 cm of sural nerve can be obtained in human clinical studies (Kim & Seo, 2001), the obtained sural nerves from cadavers and live pigs were 12.33 ± 1.2 cm and 14.5 ± 0.5 cm, respectively. The removal of the sural nerve from miniature pigs caused no functional defect. It is concluded that the sural nerve of the miniature pig can be used for peripheral nerve regeneration studies, including the examination of autologus nerve grafts with intact physiological functions. The surgical anatomy of the sural nerve of the miniature pig described in this paper would be expected to contribute to peripheral nerve reconstructive research.
Acknowledgements
The authors thank Mr. Ichirei Takeda for swine care and his swine
surgical preparation. This study was partially supported by the Global
COE program, the Multidisciplinary Education and Research Center for
Regenerative Medicine (MERCREM) of the Ministry of Education, Culture,
Sports, Science, and Technology (MEXT), Japan, and JSPS KAKENHI Grant
Number 23792386.
References
- Eom TG, GR Jeon, CM Jeong, YK Kim, SG Kim, IH Cho, YS Cho & JS Oh: Experimental study of bone response to hydroxyapatite coating implants: bone-implant contact and removal torque test. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 114, 411-418.
-
Goldwaser BR, J Magill, ME Papadaki, M Byl, R Kromann, B Yates,
LB Kaban & MJ Troulis: Continuous mandibular distraction
osteogenesis: novel device and preliminary results in minipigs.
J. Oral Maxillofac. Surg. 2013, 71, e168-e177.
-
Kim ED & JT Seo: Minimally invasive technique for sural
nerve. Harvesting: technical description and follow-up. Urology.
2001, 57, 921-924.
-
Kuo YR, CC Chen, S Goto, YT Huang, CT Wang, CC Tasi & CL
Chen: Immunomodulatory effects of bone marrow-derived
mesenchymal stem cells in a swine hemi-facial
allotransplantation model. PLoS ONE. 2012, 7, e35459.
-
Li Y, J Xu, L Mao, Y Liu, R Gao, Z Zheng, W Chen, A Le, S Shi
& S Wang: Allogeneic mesenchymal stem cell therapy for
bisphosphonate-related jaw osteonecrosis in swine. Stem Cells
Dev. 2013, 22, 2047-2056.
-
Matsumine H, R Sasaki, M Yamato, T Okano & H Sakurai: A
polylactic acid non-woven nerve conduit for facial nerve
regeneration in rats. J. Tissue Eng. Regen. Med. 2012, in
press.
-
Sasaki R, S Aoki, M Yamato, H Uchiyama, K Wada, T Okano & H
Ogiuchi: Tubulation with dental pulp cell promotes facial nerve
regeneration in rats. Tissue Eng. Part A. 2008, 14,
1141-1147.
-
Sasaki R, Y Watanabe, M Yamato, S Aoki, T Okano & T Ando:
Surgical anatomy of the swine face. Lab. Ani. 2010, 44,
359-363.
-
Sasaki R, S Aoki, M Yamato, H Uchiyama, K Wada, H Ogiuchi, T
Okano & T Ando: PLGA artificial nerve conduits with dental
pulp cells promote facial nerve regeneration. J. Tissue Eng.
Regen. Med. 2011, 5, 823-830.
-
Sasaki R, H Matsumine, Y Watanabe, M Yamato & T Ando:
Surgical anatomy of the hypoglossal nerve for facial nerve
reconstruction research in swine. J. Reconstr. Microsurg. 2014a,
30, 585–588.
-
Sasaki R, H Matsumine, Y Watanabe, Y Takeuchi, M Yamato, T
Okano, M Miyata & T Ando: Electrophysiological and
functional evaluations of regenerated facial-nerve defect with a
tube containing dental pulp cells in rats. Plastic. Reconstr.
Surg. 2014b, 134, 970-978.
-
Sasaki R, H Matsumine, Y Watanabe, M Yamato & T Ando:
Surgical procedure of extracting teeth for obtaining dental pulp
for regenerative medicine in swine. Lab. Anim. 2015, 49,
172-176.
-
Swindle MM, AC Smith, K Laber-Laird, & L Dungan: Swine in
biomedical research: management and models. ILAR news. 1994, 36,
1-5.
-
Swindle MM & AC Smith: Best practices for performing
experimental surgery in swine. J. Invest. Surg. 2013, 26,
63-71.
-
Terheyden H, S Jepsen & DR Rueger: Mandibular
reconstruction in miniature pigs with prefabricated vascularized
bone grafts using recombinant human osteogenic protein-l: a
preliminary study. Int. J. Oral Maxillofac. Surg. 1999, 28,
461-463.
-
Vodicka P, K Smetana Jr, B Dvorankova, T Emerick, Y Xu, J
Ourednik, V Ourednik & J Motlik: The miniature pig as an
animal model in biomedical research. Ann. NY Acad. Sci. 2005,
1049, 161-171.
- Wang S, Y Liu, D Fang & S Shi: The miniature pig: a useful large animal model for dental and orofacial research. Oral Dis. 2007, 13, 530-537.