Double Decker Enrichment cages have no effect on long term nociception in neuropathic rats but increase exploration while decreasing anxiety-like behaviors
by Pascal Vachon
 Correspondence: Pascal Vachon
Correspondence: Pascal Vachon
University of Montreal, Faculty of Veterinary Medicine, Department of
Veterinary Biomedicine,
Saint-Hyacinthe, Québec, Canada J2S 2M2
Tel + 1 (450) 773-8521 ext. 8294
E-mail pascal.vachon@umontreal.ca
Summary
In this present study, we investigated the impact of environmental enrichment in Sprague Dawley rats up to three months after a chronic or sham nerve injury. Sprague Dawley rats were housed in either standard polycarbonate cages or rat enrichment cages. Following 2 weeks of training and the recording of baseline behavioral values, half of the animals underwent a right sciatic nerve chronic constriction injury (CCI) surgery under general anaesthesia to induce chronic neuropathic pain. The other animals underwent a sham surgery. Animals were then evaluated once a month for 3 consecutive months in different behavioural tests for of mechanical and heat sensitivities as well as for exploration and anxiety-like behaviors. Mechanical and heat sensitivities were also tested at 15 days following the surgery. One month following the surgery, half of the rats in each group (CCI and sham) were either left in the standard rat cages or placed in the Double Decker cages. Environmental enrichment did not affect the mechanical or heat sensitivity of neuropathic animals; however exploration increased, and anxiety-like behaviours decreased, significantly (p<0.01). These results clearly show that environmental enrichment can have a significant impact on exploratory and anxiety-like behaviour in neuropathic rats without modifying pain hypersensitivity.
Introduction
Enrichment of both social and physical life is gaining interest for
the treatment of chronic pain, since an enriched environment may be
protective against the development of chronic pain. For example,
studies in animals (Tagerian et al., 2013; Vachon et al., 2013; Gabriel et al., 2009,
2010a, 2010b; Tall 2009) and humans (Smith et al., 2003; Ulrich, 1984) looking at
the effects of the environment on pain recovery following surgery
showed a significant alleviating effect with environmental enrichment.
Environmental enrichment may also protect against the development of
depression and anxiety disorders, both of which are commonly
associated with chronic pain in animals (Tagerian et al., 2013; Seminowicz et al., 2009; Suzuki et al.,
2007) and humans (Campbell et al., 2003; Daniel et al., 2008; Dick et al., 2008;
Haythornthwaite et al., 2000; Kewman et al., 1991; Petrak et al.,
2003). Social isolation on the other hand has unclear effects on pain
sensitivity. Adler et al. (1975) and DeFeudis
et al. (1976) reported that isolation had no effect on pain
behaviors; however Panksepp (1980) suggested that it may increase pain
sensitivity.
Rodents living in enriched environments show less pain behavior
following a peripheral nerve or central nervous system injury than
those living in standard or restricted environments (Tall, 2009; Gabriel et al., 2009; 2010a; 2010b, Berrocal et al.,
2007; Lankhorst et al., 2001). However in these studies animals were kept in the enriched
environment from the time of injury onward. Most studies have been
conducted with healthy non-injured animals (Chourbadji et al., 2008; Rossi & Neubert, 2008; Smith et al.,
2003, 2004 & 2005) as well as animals with inflammatory (Gabriel et al., 2009; 2010a,b; Tall, 2009) and neuropathic pain (Stagg et al., 2011). It has recently
been shown that pain can be alleviated by environmental enrichment
well after the establishment of chronic pain in mice (Vachon et al., 2013; Tajerian et al., 2013). In this study, we investigated whether leaving neuropathic rats in
standard polycarbonate cages or putting neuropathic rats into
enrichment cages one month after surgery would alter pain sensation as
well as associated anxiety-like and exploratory behaviors.
Materials and methods
Animals and husbandry
Twenty-four Sprague Dawley rats (CRL:CD[SD]; Charles River,
St-Constant, Canada) weighing between 225-250 g were purchased for
this study. Following their arrival, they were kept in a standard
laboratory animal environment (fresh filtered air, 15 changes/ hour;
temperature, 21 ± 2oC; humidity 50 ± 20%; and light-dark cycle, 12:12h). Rats were housed on hardwood bedding (Beta
chip, Northeastern Products, USA) with only black PVC tubing for
environmental enrichment and they were placed 2 per cage in large
polycarbonate cages (48x38x21 cm; Tecniplast) up to one month
following surgery. Rats received tap water and a standard laboratory
rodent diet (Charles River Rodent Chow 5075, Canada)
ad libitum. The Faculty of Veterinary Medicine Institutional
Animal Care and Use Committee approved the experimental protocol prior
to animal use in accordance with the guidelines of the Canadian
Council on Animal Care (1993).
Following their arrival and a one-week acclimation period, rats were
trained for two weeks to the different behavioral tests and baseline
values were taken. A peripheral mononeuropathy was then produced in 12
animals using the chronic sciatic nerve constriction (CCI) model (Bennett and Xie,
1988). Briefly, following anesthesia with isoflurane
(AErrane; Baxter), the right common sciatic nerve was exposed via
blunt dissection at the level of the thigh (biceps femoris).
Four loose ligatures were placed around the right sciatic nerve with
4.0 Catgut suture material. The overlying muscles were then sutured
with 3.0-vicryl and the skin was closed with a 2.0 silk suture.
Animals were then tested in all behavioral tests once a month for 3
consecutive months following the surgery. In addition, mechanical (von
Frey test) and heat sensitivity (Hargreaves test) were also evaluated
15 days following the surgery. The other 12 animals underwent a sham
surgery (same procedures without the sciatic ligatures).
One month following surgery (and behavioral evaluations at one month),
CCI rats were randomly assigned to either of 2 groups: 1) nerve injury
and environmental enrichment (NE, n=6) or 2) nerve injury and standard
housing (NS, n=6), while sham animals were assigned to either 1) sham
surgery in environmental enrichment (SE, n=6) or 2) sham surgery and
standard housing (SS, n=6). Environmental enrichment consisted of
Double Decker enrichment cages (Tecniplast Inc.) and for the standard
housing animals were left in the large polycarbonate cages.
Behavioral evaluations
For all behavioral tests, each apparatus was washed with a very
diluted cleaning solution between two consecutive animals to minimize
odor interference.
Mechanical sensitivity Calibrated von Frey filaments
(Stoelting Co.) were applied for 4 sec or until withdrawal. The
stimulus force ranged from 1 - 26 g, corresponding to filament sizes
4.08 – 5.46 g. For each animal, the actual filaments used within the
aforementioned series were determined based on the lowest filament to
evoke a positive response using the up-down method. Animals were
acclimated to the experimental setup for 15 minutes prior to testing.
The mechanical sensitivity was assessed on the plantar surface of both
hind paws.
Hargreaves thermal sensitivity test: Thermal sensitivity was
evaluated using a Hargreaves apparatus (IITC Life Science) as
previously described (Hargreaves et al., 1988). Each animal
was placed in a Plexiglas chamber with the ground floor made of heated
glass (29-31°C). Animals were allowed to acclimate to the experimental
set up for 15 min prior to testing. Then radiant heat generated by a
high intensity light bulb (40W) was directed to the plantar surface of
a hind paw. The lamp generated noxious a heat stimulus. The time the
animal took to lift its paw from the floor was recorded and noted as
the thermal threshold. Rats were tested in groups of 4 animals. The
test began alternatively with the right or the left hind paw to
prevent any anticipatory behavior. A cut-off time for the radiant
stimulation was set at 20 sec to minimize tissue injury.
Open field test: Spontaneous exploratory
behavior was evaluated in a transparent open field apparatus (60 x 60
cm), placed in a quiet room. The floor of the apparatus was divided
equally into nine squares (20 x 20 cm2). Rats were individually placed
into the open field on the central square, and their spontaneous
behavior was videotaped for 5 minutes before being scored by an
observer blinded to the experimental protocol. Subsequent analysis of
the total number of squares visited, as well as the number of times
rats stood on their hind paws, was used to assess general motor
activity and exploration.
Elevated plus maze: An elevated plus maze
apparatus was placed in a quiet room. The apparatus consisted of two
open arms (100 cm long x 10 cm wide) across from each other and
perpendicular to two closed arms (2 x 45 cm long x 10 cm wide x 30 cm
high). The entire apparatus was positioned 60 cm above the floor. Rats
were individually placed at the intersection of the four arms, head
towards an open arm. Their spontaneous behavior was videotaped for 5
minutes before being scored by a blinded observer. The total time
where partial (at least two front paws in contact with the open arm)
or total body was on an open arm was measured.
Statistical analysis
A 2-way repeated measures ANOVA was performed for all behavioral
tests. Post hoc Tukey tests were performed to assess the
difference between groups at different time points. The level of
significance was set at 0.05. Data are presented as mean
± SD .
Results
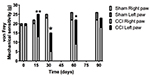
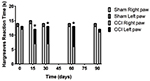
The von Frey filaments and Hargreaves results are presented in s 1
& 2. Results from non-enriched, as well as enriched, environments
were pooled together since no significant differences occurred with
environmental conditions. Only the ipsilateral hind paw of
nerve-injured animals was hypersensitive to mechanical stimuli up to
60 days following the surgery (p<0.05 at 15 days and p<0.01 at
30 & 60 days). The same general findings were obtained for the
Hargreaves test (p<0.01 at 15, 30 & 60 days for the ipsilateral
hind paw in CCI animals). At 90 days following the surgery, CCI
animals were no longer neuropathic for either mechanical or heat
sensitivities.
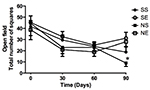
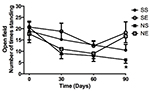
When looking at the total number of squares entered in the open field
test (Figure 3) and the number of times the animals stood on their
hind paws (Figure 4) over a 5 minute period, there were no differences
between the experimental groups up to 60 days, however a decrease in
exploration occurred over time, which could be associated with
learning. At 90 days a clear difference in both behaviors occurred for
the neuropathic animals: enrichment cages had a clear benefit over the
standard environment (p<0.01). Although not significant, a trend to
increased exploration and standing behavior at 90 days occurred for
sham animals placed in enrichment cages.
With the elevated plus maze (Figure 5) all groups explored the open
arm for the same percentage of time up the 30 days post-surgery. At 60
days, sham animals in enriched environment were clearly less anxious
than the sham animals in the standard environment (p<0.01), with no
significant difference seen amongst other groups. At 90 days
post-surgery, neuropathic animals in the enriched environment clearly
showed less anxiety-like behavior (greater exploration of the open
arm) than neuropathic animals in the standard environment (p<0.01).
Discussion
Our results clearly show that environmental enrichment has a
significant impact on exploratory and anxiety-like behaviors, but
doesn’t affect pain hypersensitivity in a rat model of
neuropathic pain. At 60 days post-surgery, environmental enrichment
did not affect pain threshold to mechanical and heat stimuli in rats,
an effect that was clearly seen in mice (Vachon et al, 2013).
At 90 days post-surgery, the loss of heat and mechanical pain
sensitivities in neuropathic animals is a normal finding when using
the CCI model (Coggeshal et al, 1993). Interestingly these
animals benefited the most from the enrichment cages (decreased
anxiety and increased exploration) even though they were no longer
neuropathic. Since standing and exploratory behaviors increased, and
anxiety-like behaviour decreased, in neuropathic animals when housed
in the Double Decker cages, this strongly suggests that their
well-being increased. Importantly, cage size would appear to influence
some higher brain functions without affecting chronic pain processing.
We have previously shown (Vachon et al., 2013; Tajerian et al., 2013) that environmental enrichment in mice had an alleviating effect on
well established chronic neuropathic pain and anxiety-like behaviors.
One of the main components of the enriched environment in this
previous study was a running wheel, and exercise is known to decrease
pain symptoms and improve motor function in chronic pain models. Stagg
et al. (2011), demonstrated that thermal hyperalgesia in a
rat model of neuropathic pain is reversed with exercise.
Interestingly, reversal of sensory hypersensitivity was seen even when
exercise was initiated 4 weeks after spinal nerve ligation, and
returned 5 days after discontinuing exercise. In the present study,
the main focus was the effect of cage size on pain-related behaviors
and therefore the addition of exercise toys could very well provide an
additional benefit, if the objective is to treat pain. Exercise
appears sufficient to have a significant alleviating effect on
neuropathic pain symptoms, and clinical studies in humans suggest that
exercise decreases pain symptoms in chronic pain patients (Malmros et al., 1988; Ferrel et al., 1997; Gowan et al., 2004;
Hayden et al., 2005; Robb et al., 2006; Chatzitheodorou et al.,
2007).
In conclusion, the present findings suggest that environmental
enrichment cages appear to be a very interesting option when studying
neuropathic pain in a rat model, since pain behaviors didn’t
appear to be affected but there was evidence for increased animal
welfare.
Acknowledgements
This work was made possible by the financial support of the Laboratory Animal Research Fund (Dr Pascal Vachon research lab), the donation of Double Decker cages from Tecniplast, the technical and material support (Tecniplast standard rat cages) of Ste-Justine Hospital Research Centre, and Dr Geneviève Roy who did all video data acquisition.
References
-
Adler MW, C Mauron, R Samanin &, L Valzelli: Morphine
analgesia in grouped and isolated rats. Psychopharmacologia 1975,
41, 11-14.
-
Bennett GJ & YK Xie: A peripheral mononeuropathy in rat
produces disorders of pain sensation like those seen in man. Pain
1988, 33, 87-107.
-
Berrocal Y, DD Pearse, A Singh, CM Andrade, JS McBroom, R Pentes
& MJ Eaton: 2007. Social and environmental enrichment
improves sensory and motor recovery after severe contusive spinal
cord injury in the rat. J. Neurotrauma 2007, 24, 1761-1772.
-
Campbell LC, DJ Clauw & FJ Keefe: Persistent pain and
depression, a biopsychosocial perspective. Biol. Psychiatry 2003,
54, 399-409.
-
Canadian Council on Animal Care: Guide to the Care and Use
of Experimental Animals. Ottawa, Ontario, Canada, 1993.
-
Chatzitheodorou D, C Kabitsis, P Malliou & V Mougios: A
pilot study of the effects of high intensity aerobic exercise versus
passive interventions onpain, disability, psychological strain, and
serum cortisol concentrations in people with chronic low back pain.
Phys. Ther. 2007, 87, 304-312.
-
Chourbaji S, C Brandwein, MA Vogt, C Dormann, R Hellweg & P
Gass : Nature vs nuture, can enrichement rescue the behavioral phenotype
of BDNF heterozygous mice ? Behav. Brain Res. 2008, 192, 254-258.
-
Coggeshall RE, PM Dougherty, CM Pover & CM Carlton:. Is
large myelinated fiber loss associated with hyperalgesia in a model
of experimental peripheral neuropathy in the rats. Pain 1993, 52:
233-242
-
Daniel HC, J Narewska, M Serpell, B Hoggart, R Johnson & ASC
Rice:
Comparison of psychological and physical function in neuropathic
pain and nociceptive pain, implications for cognitive behavioral
pain management programs. Eur. J. Pain 2008, 12, 731-741.
-
DeFeudis FV, PA DeFeudis & E Somoza: Altered analgesic
responses to morphine in differentially housed mice.
Psychopharmacol. 1976, 49, 117-118.
-
Dick BD, MJ Verrier, KT Harker & S Rashiq: Disruption
of cognitive function in fibromyalgia syndrome. Pain 2008, 139,
610-616.
-
Ferrel BA, KR Josephson, AM Pollan, S Loy & BR Ferrell: A randomnized trial of walking versus physical methods for chronic
pain management. Aging 1997, 9, 99-105.
-
Gabriel AF, MAE Marcus, WNM Honig, N Helgers & EA
Joosten:
Environmental housing affects the duration of mechanical allodynia
and the spinal astroglial activation in a rat model of chronic
inflammatory pain. Brain Res. 2009, 1276, 83-90.
-
Gabriel AF, G Paoletti, D Della Seta, R Panelli, MAE Marcus, F
Farabollini, G Carli & EA Joosten:
Enriched environment and the recoevry from inflammatory pain, Social
versus physical aspects and their interaction. Behav. Brain Res.
2010a, 208, 90-95.
-
Gabriel AF, MAE Marcus, WNM Honig & EA Joosten:
Preoperative housing in an enriched environment significantly
reduces the duration of post-operative pain in a rat model of knee
inflammation. Neurosci. Lett. 2010b, 469, 219-223.
-
Gowan SE, A Dehueck, B Voss, A Silaj & SE Abbey:
Six-month and one-year follow-up of 23 weeks of aerobic exercise for
individuals with fibromyalgia. Arthritis Rheum. 2004, 51,
890-898.
-
Hargreaves K, R Dubner, F Brown, C Flores & J Joris: A
new and sensitive method for measuring thermal nociception in
cutaneous hyperalgesia. Pain 1998, 32, 77−88.
-
Kewman DG, N Vaishampayan, D Zald & B Han: Cognitive
impairment in musculoskeletal pain patients. Int. J. Psychiatry Med.
1991, 21, 253-262.
-
Lankhorst AJ,MP ter Laak,TJ van Laar ,NL van Meeteren, JC de
Groot & LH Sharma: Effects of enriched housing on functional recovery after spinal
cord contusion injury in the adult rat. J. Neurotrauma 2001, 18,
203-215.
-
Malmros B, L Mortensen, MB Jensen & P Charles : Positive effecs of
physiotherapy on chronic pain and performance in osteoporosis.
Osteoporos. Int. 1998, 8, 215-221.
-
Panksepp J: Brief social isolation, pain responsitivity,
and morphine analgesia. Psychopharmacol. 1980, 72, 111-112.
-
Petrak F, J Hardt, B Kappis, R Nickel & EU Tiber :
Determinants of health-related quality of life in patients with
persistent somatoform pain disorder. Eur. J. Pain 2003, 7,
463-471.
-
Robb KA, JE Williams, V Duvivier & DJ Newham DJ: A pain
management program for chronic cancer-treatment-related pain, A
preliminary study. J. Pain 2006, 7, 82-90.
-
Rossi HL & JK Neubert: Effects of environmental
enrichement on thermal sensitivity in an operant orofacial pain
assay. Behav. Brain Res. 2008, 187, 478-482.
-
Seminowicz DA, AL Laferriere, M Millecamps, JSC Yu, TJ Coderre
& MC Bushnell:. MRI structural brain changes associated with sensory and
emotional function in a rat model of long-term neuropathic pain.
NeuroImage 2009, 47, 1007-14.
-
Smith MA, PA Bryant & McClean: Social and environmental
enrichment enhances sensitivity to the effects of kappa
opioids , studies on antinociception, diuresis and conditioned
place preference. Pharmacol. Biochem. Behav. 2003, 76, 93-101.
-
Smith MA, JM McClean & PA Bryant: Sensitivity to the
effects of a kappa opioid in rats with free access to exercise
wheels, differential effects acrsoos behavioral measures. Pharmacol.
Biochem. Behav. 2004, 77, 49-57.
-
Smith MA, KA Chislom, PA Bryant, JL Greene, JM McClean, WW Stoops
& DL Yancey: Social and environmental influences on opioid sensitivity in rats,
importance of an opioid’s relative efficacy at the
mu-receptor. Psychopharmacol. 2005, 181, 27-37.
-
Stagg NJ, HP Mata , MM Ibrahim, EJ Henriksen, F Porecca, TW
Vanderah & MT Philip: Regular exercise reverses sensory hypersensitivity in a rat
neuropathic pain model, role of endogenous opioids. Anesthesiology
2011, 114, 940-48.
-
Suzuki T, M Amata, G Sakaue, S Nishimura, T Inoue, M Shibata
& T Mahimo : Experimental neuropathy in mice is associated with delayed
changes related to anxiety and depression. Anesth. Analg. 2007, 104,
1570-1577.
-
Tajerian M, S Alvarado, M Millecamps, P Vachon, C Crosby, MC
Bushnell, M Szyf & LS Stone:
Peripheral nerve injury is associated with chronic, reversible
changes in global DNA methylation in the mouse prefrontal cortex.
Plos One 2013, 8 e55259.
-
Tall JM : Housing supplementation decreases the magnitude
of inflammation-induced nociception in rats. Behav. Brain Res. 2009,
197, 230-33.
-
Ulrich RS: View through a window may influence recovery
from surgery. Science 1984, 224, 420-421.
Vachon P, M Millecamps, LA Low, SJ Thompson, F Pailleux, F Beaudry, MC Bushnell & LS Stone: Alleviation of chronic neuropatnic pain by environmental enrichment in mice well after the establishment of chronic pain. Behav. Brain Funct. 2013, 9,22.