Correlation between synthesis of α2-macroglobulin in hepatocytes and changes in serum cytokine levels in rats after infl ammatory stimulation
by Takashi Kuribayashi1, Tetsurou Seitα1, Eiichi Momotani2, Toshio Honjo3, Shunsuke Yamazaki4 & Shizuo Yamamoto1
1Laboratory of Immunology, School of Life and
Environmental Science, Azabu University, Sagamihara, Kanagawa,
Japan
2Research Team for Paratuberculosis, National Institute
of Animal Health, Tsukuba, Ibaraki, Japan
3Seminar on Environment, School of Life and Environmental
Science, Azabu University, Sagamihara, Kanagawa, Japan
4Laboratory of Physiology, Department of Food and
Nutrition, Kamakura Women’s University, Kamakura, Kanagawa,
Japan
 Correspondence: Shizuo Yamamoto
Correspondence: Shizuo Yamamoto
Laboratory of Immunology, School of Life and Environmental Science
Azabu University
1-17-71 Fuchinobe, Chuo-ku, Sagamihara, Kanagawa 252-5201, Japan
Tel +81-42-754-7111,
Fax +81-42-754-2461,
E-mail kuribaya@azabu-u.ac.jp
Summary
The time course of α2-macroglobulin (α2M) synthesis in rat liver was investigated using immunohistochemistry. Furthermore, correlations between synthesis of α2M in hepatocytes and interleukin (IL)-6 and cytokine-induced neutrophil chemoattractant-1 (CINC-1), which are considered to contribute to the production of α2M, were evaluated. The presence of α2M in the liver was investigated by immunohistochemistry, and serum levels of α2M, IL-6 and CINC-1 were measured by enzyme-linked immunosorbent assay. α2M was not detected in the liver before injection of turpentine oil. α2M was detected throughout the liver at 12 hours after injection of turpentine oil, when high serum levels of IL-6 and CINC-1 were observed. α2M was distributed mainly around the central vein of the liver at 36 hours. Only small amounts of α2M were detected in the liver at 48 hours, when peak serum levels of α2M were observed. Synthesis of α2M in hepatocytes peaked long before peak α2M serum levels were seen. In conclusion, α2M was considered to be synthesized in response to stimulation by IL-6 and CINC-1.
Introduction
Acute-phase proteins are useful inflammatory markers due to their obvious increases during bacterial infections and after surgical treatments as well as their changes in serum levels under disease conditions (Ceron et al., 2005; Jinbo et al., 2002; Honjo et al., 2010; Kuribayashi et al., 2003; Lazovic, 2012; Pepys et al., 1983). C-reactive protein (CRP) is a typical acute-phase protein in humans and dogs (Capsi et al., 1984; Castell et al., 1988; Ceron et al., 2005; Du Clos, 2000; Eckersall et al., 1993; 1994; Karadag et al., 2008; Morris et al., 1982, Rahman et al., 2008). On the other hand, α2-macroglobulin (α2M) and α1-acid glycoprotein (AAG) are typical acute-phase proteins in rats (Honjo et al., 2010; Honjo et al., 2010; Inoue et al., 2001; Jinbo et al., 2001; Jinbo et al., 2002). Serum levels of α2M and AAG increase in rats after injection of turpentine oil, surgical treatment or inoculation with Staphylococcus aureus (Honjo et al., 2010). However, α2M reacts more sensitively than AAG in rats during acute inflammation. Thus, α2M is considered to be more useful than AAG as an acute-phase protein in rats.
α2M is produced in the liver (Du Clos, 2000; Heinrich et al., 1990), and interleukin (IL)-6 and cytokine-induced neutrophil chemoattractant-1 (CINC-1) are thought to contribute to its production (Baumann et al., 1989; Ling et al., 2004; Nijsten et al., 1987; Richard et al., 2008; Sheikh et al., 2006). Changes in serum α2M levels have been widely investigated and the kinetics of serum α2M have been clarified (Honjo et al., 2006; Honjo et al., 2010; Jinbo et al., 2001; Jinbo et al., 2002; Kuribayashi et al., 2011; Kuribayashi et al., 2012). However, the time course of α2M synthesis in rat liver has not been investigated using immunohistochemistry. Furthermore, correlations between α2M synthesis and changes in serum levels of α2M, IL-6 and CINC-1 have not been clarified. In the present study, the time course of α2M synthesis in rat liver after acute inflammatory stimulation was investigated by immunohistochemistry. Moreover, correlations between α2M synthesis in the liver and changes in serum levels of α2M, IL-6 and CINC-1 were evaluated.
Methods
Animals
Twenty-four male Sprague-Dawley rats (age, 9 weeks) were purchased from Charles River Laboratories Japan (Yokohama, Kanagawa, Japan). Rats were kept in cages at a temperature of 23 ± 2°C, and a relative humidity of 55% ± 10%, on a 12/12 dark (18:00-6:00)/light (6:00-18:00) cycle with the air exchanged 12 times or more per hour. Rats were fed MF (Oriental Yeast Co., Ltd., Tokyo, Japan), and were allowed free access to water. All experiments conformed to Japanese regulations concerning animal care and use, as described in the Guidelines for Animal Experimentation (Japanese Association for Laboratory Animal Science, JALAS, 1987). The present animal experiment was approved by the Institutional Animal Care and Use Committee of Azabu University.
Animal experimental design
Turpentine oil (Wako Pure Chemical Industries, Co., Ltd., Osaka,
Japan) was intramuscularly injected at 0.4 ml/rat. Rats were
sacrificed under anesthesia with an intravenous injection of
pentobarbital (Kyoritsu Seiyaku Corporation, Tokyo, Japan) before
treatment and at 6, 12, 18, 24, 36, 48 or 72 hours after injection of
turpentine oil. Three rats were sacrificed at each time point.
Unfortunately, liver samples could not be collected from the same rat
at each time point, as pricking with the biopsy needle would
constitute inflammatory stimulation and would influence the kinetics
of α2M. Thus, whole livers were collected at each time point from
different rats. Blood was collected from the aorta when the liver was
removed. Sera were obtained by centrifugation (1,600 × g, 15
minutes), and were stored at -80°C until measurement of α2M, IL-6 and
CINC-1.
Immunohistochemistry
Liver samples were fixed in 10% formalin and embedded in paraffin.
Sections (3 mm) were cut, incubated with Blocking One (Nacalai Tesque,
Inc., Kyoto, Japan) diluted with 0.01 M phosphate buffered saline for
15 minutes. Next, mouse anti-α2M monoclonal antibody (Inoue et al., 2001) was applied for 1 hour. Sections were stained using the
peroxidase method with Histofine Simple Stain Rat MAX-PO(M) (Nichirei
Biosciences Inc., Tokyo, Japan) and Simple Stain DAB solution
(Nichirei Biosciences Inc.).
Measurement of α2M, IL-6 and CINC-1
Serum levels of α2M were measured by enzyme-linked immunosorbent assay
(ELISA) according to the procedure of Honjo et al. (2010).
Serum levels of IL-6 and CINC-1 were measured by ELISA using
commercial kits. Commercial kits for IL-6 and CINC-1 were purchased
from BioSource International, Inc. (Camarillo, CA, USA) and Panapharm
Laboratories Co., Ltd. (Kumamoto, Japan), respectively.
Serum levels at each point are given as means ± standard deviation.
Results
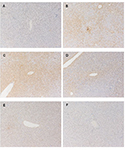
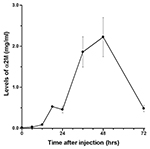
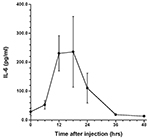
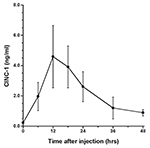
The presence of α2M in rat liver after acute inflammatory stimulation is shown in Figure 1. Similar observations were obtained from other rats at each time point. Changes in serum levels of α2M are shown in Figure 2, while changes in serum levels of IL-6 and CINC-1 are shown in Figures 3 and 4, respectively.
α2M was not detected in the liver before treatment, but α2M was detected particularly in perilobular hepatocytes at 6 hours after injection of turpentine oil. The presence of α2M was observed in hepatocytes throughout the liver at 12 hours, and was observed mainly around the central veins in the liver at 24 hours. Small amounts of α2M were observed throughout the liver at 48 hours, and α2M was no longer observed in the liver at 72 hours after injection.
The peak serum level of α2M was 2.2±0.48 mg/ml at 48 hours after turpentine injection, after which serum levels decreased. The peak levels of IL-6 and CINC-1 were 235.6±122.3 pg/ml at 18 hours and 4.6±2.1 ng/ml at 12 hours after turpentine oil injection, respectively.
Discussion
The time course of α2M synthesis in rat liver was investigated using immunohistochemistry. The pattern of changes in serum α2M was similar to previous data obtained in individual rats after inflammatory stimulation, with peak serum levels observed at 48 hours after turpentine oil injection (Jinbo et al., 2001; Jinbo et al., 2002). Synthesis of CRP in rabbits has been evaluated using an immunoenzymatic technique (Macintyre et al., 1982), and CRP was not detected in hepatocytes of rabbits not injected with turpentine oil. Similarly, in the present study, α2M was not detected in the liver prior to turpentine oil injection, despite small amounts of α2M being present serum prior to inflammatory stimulation. α2M was observed particularly in perilobular areas of the liver at 6 hours after turpentine oil injection, and subsequently increasing numbers of hepatocytes produced α2M around the central veins of liver. These synthesis patterns of α2M in rats were similar to those of CRP in rabbits. α2M was detected throughout the liver of rats at 12 hours, when mean serum levels of IL-6 and CINC-1 showed high levels. However, α2M levels in serum were very low (0.09 mg/ml)at 12 hours. On the other hand, α2M was weakly detected in hepatocytes at 48 hours, when peak serum levels of α2M were observed.
Jinbo et al. investigated serum changes of α2M and several cytokines in rats after injection of turpentine oil and found that only IL-6 and CINC-1 increased prior to elevation of α2M (Jinbo et al., 2002). We believe that IL-6 and CINC-1 contribute to synthesis of α2M in rats (Honjo et al., 2006; Honjo et al., 2010; Jinbo et al., 2002; Ling et al., 2004; Richard et al., 1991; Sheikh et al., 2006; Kuribayashi et al., 2011). Correlations between synthesis of α2M in the liver and changes in IL-6 and CINC-1 in serum were thus evaluated in this study. Circulating blood enters the liver lobules from interlobular arteries and veins through the sinusoidal capillaries and flows into the central vein of each lobule. Honjo et al. (2010) reported that serum levels of α2M were elevated after injection of a mixture of IL-6- and CINC-1-rich fractions separated from rat sera after acute inflammatory stimulation; however, α2M was not elevated after individual injection of IL-6- or CINC-1-rich fraction. Peak synthesis of α2M in hepatocytes corresponded to peak serum levels of IL-6 and CINC-1. This suggests that both IL-6 and CINC-1 are involved in signaling hepatocytes to synthesize α2M.
Acknowledgements
This research was partially supported by The Promotion and Mutual Aid Corporation for Private Schools of Japan, and a Grant-in-Aid for Matching Fund Subsidy for Private Universities.
References
-
Baumann H, KR Prowse & S Marinkovic: Stimulation of
hepatic acute phase response by cytokines an glucocorticoids. Ann NY
Acad Med Sci, 1989, 557, 180-195.
-
Capsi D, ML Baltz, F Snel, E Gruys, D Niv, RM Batt, EA Munn, N
Buttress & MB Pepys:Isolation and Characterization of C-reactive protein from the dog.
Immunology, 1984, 53, 307-313.
-
Castell JV, MJ Gomez-Lechon, D Martina, T Hirano, T Kishimoto
& PC Heinrich:Recombinant human interleukin-6(IL-6/BSF-2/HSF) regulates the
synthesis of acute phase proteins in human hepatocytes.
FEBS, 1988, 232, 347-350.
-
Ceron JJ, PD Eckersall & S Martinez-Subiela: Acute
phase in dogs and cats: current knowledge and future perspectives.
Vet Clin Pathol, 34, 2005, 85-99.
-
Du Clos TW :Function of C-reactive protein. Ann Med,
2000, 32, 274-278.
Eckersall PD, MJA Harvey, JM Ferguson, JP Renton, DA Nickson & JS Boyd:Acute phase proteins in canine pregnancy (Canis familiaris). J Reprod Fertil, 1993, 47, 159-164.
-
Eckersall PD, S Duthie, M Toussaint, E Gruys, P Heegaars, M Alava
C Lipperheide & F Madec: Standarization of diagnostic assays for animal acute phase
proteins.Adv Vet Med, 1994, 41, 643-655.
-
Heinrich PC, JV Castell & T Andus: Interleukin-6 and
acute phase response. Biochem J, 1990, 265,
621-636.
-
Honjo T, T Kuribayashi, T Seita, K Tamura, M Matsunoto, H
Seguchi, K Ogihara, S Yamazaki & S Yamamoto: Variations in α1-acid glycoprotein (α1*AG) and inflammatory
cytokines (IL-6, CINC-1) in healthy rats following acute
inflammation - α1-acid glycoprotein and cytokines in rats -.
Vet Biochem,2006, 43, 9-15.
-
Honjo T, T Kuribayashi, M Matsumoto, S Yamazaki & S
Yamamoto:Kinetics of α2-macrogobulin and α1-acid glycoprotein in rats
subjected to repeated acute inflammatory stimulation. Lab Anim,2010,
44, 150-154.
-
Honjo T, T Kuribayashi, Y Mokonuma, A Yamaga, S Yamazaki & S
Yamamoto: The effects of interleukin-6 and cytokine-induced neutrophil
chemoattractant-1 on α2-macroglobulin production in rat.Exp
Anim,2010, 59, 589-594.
-
Inoue S, T Jinbo, M Shino, K Iguchi, M Nomura, K Kawato & S
Yamamoto: Determination of α2-macroglobulin concentrations in healthy rats
of various ages and rats inoculated with turpentine oil by
enzyme-linked immunosorbent assay. J Exp Anim Sci, 2001,
42, 44-49.
-
Jinbo T, M Motoki & S Yamamoto: Variation of serum
α2-macroglobulin concentration in healthy rats and rats inoculated
Staphylococcus aureus or subjected to surgery. Comp Med,
2001, 51, 332-335.
-
Jinbo T, T Sakamoto & S Yamamoto: Serum
α2-macroglobulin and cytokine measurements in an cute inflammation
model in rats. Lab Anim, 2002, 36, 153-157.
-
Karadag F, S Kirdar, AB Karul & E Ceylan: The value of
C-reactive protein as a marker of systemic inflammation in stable
chronic obstructive pulmonary disease. Eur J Int Med, 2008,
19, 104-108.
-
Kuribayashi T, T Shimada, M Matsumoto, K Kawato, T Honjo, M
Fukuyama, Y Yamamoto & S Yamamoto: Determination of serum C-reactive protein (CRP) in healthy beagle
dogs of various ages and pregnant beagle dogs. Exp Anim,
2003, 52, 387-390.
-
Kuribayashi T, M Tomizawa, T Seita, K Tagata & S Yamamoto; Relationshipn between production of acute-phase proteins and
strength of inflammatory stimulation in rats. Lab Anim,
2011, 45, 215-218.
-
Kuribayashi T, T Seita, T Honjo, S Yamazaki, E Momotani & S
Yamamoto; Impairment of α2-macroglobulin synthesis in experimental
hepatopathic rats treated with turpentine oil, Exp Anim,
2012, 61, 125-130.
-
Lazovic B: Correlation of CRP and serum levels fibriinogen
with severity of disease in chronic obstructive pulmonary disease
patients. Med Arh, 2012, 66, 159-60.
-
Ling PP, RJ Smith, S Kie, P Boyce & BR Bistrian:
Effects of protein malnutrition on IL-6-mediated signaling in the
liver and the systemic acute-phase response in rats. Am J Physiol
Regul Integr Comp Physiol,2004, 287, 801-808.
-
Macintyre SS, D Schultz & I Kushner: Biosynthesis of
C-reactive protein. Ann NY Acad Med Sci, 1982, 389,
76-87.
-
Morris SG, D Gomez & KR Prasad: C-reactive protein in
liver cancer surgery.Eur J Surg Oncol, 2008, 34,
727-729.
-
Nijsten
MWN, ER DeGroot, HJ TenDuis, JH Klaren, CE Hack & LA
Arden: Serum levels of interleukin-6 and acute phase respose.
Lancet, 1987, 330, 921.
-
Pepys MB & ML Baltz: Acute-phase proteins with special
reference to C-reactive protein and related proteins (pentaxins) and
serum amyloid A protein. Adv Immunol, 1983, 34,
141-212.
-
Rahman SH, J Evans, GJ Toogood, PA Lodge & KR Prasad:
Prognostic utility of postoperative C-reactive protein for
posthepatectomy liver failure. Arch Surg, 2008, 143,
247-253.
-
Richard C, J Gauldie & H Baumman:
Cytokinecontrol of acute phase protein expression. Eur Cytokien
Netw, 1991, 2, 89-298.
- Sheikh N, K Tron, J Dudas & G Ramadori: Cytokine induced neutrophil chmoattractant-1 is released by the noninjured liver in a rat acute phase model. Lab Invest, 2006, 86, 800-814.