Cerebral protection for the preclinical evaluation of a vascular graft in sheep carotid artery model
by Harikrishnan,V.S. and Umashankar, P.R.
Biomedical Technology Wing, Sree Chitra Tirunal Institute for
Medical Science and Technology, Thiruvananthapuram, Kerala,
India
 Correspondence: Dr. P.R. Umashankar, Scientist E, Division of In vivo Models and
Testing, Biomedical Technology Wing, Sree Chitra Tirunal Institute for
Medical Science and Technology, Poojappura Thiruvananthapuram, Kerala,
India, PIN 695012.
Correspondence: Dr. P.R. Umashankar, Scientist E, Division of In vivo Models and
Testing, Biomedical Technology Wing, Sree Chitra Tirunal Institute for
Medical Science and Technology, Poojappura Thiruvananthapuram, Kerala,
India, PIN 695012.
Email: umashnkr@sctimst.ac.in
Telephone: +91 471 2520226 (off.), +91 9495984231 (mob.)
Fax: +91 471 2341 814
Summary
A protocol for cerebral protection without systemic hypothermia to aid the safe, smooth and fast recovery of sheep used for the preclinical evaluation of a prosthetic vascular graft in the carotid artery is presented in this study. Ten adult Ramnad white sheep (33.8± 3.2 kg) were green grass deprived and anticoagulated from 5 days prior to surgery with aspirin 150 mg and clopidogrel 75 mg till the end of the study. After anesthetic premedication and endotracheal intubation, the animals were ventilated at the rate of 12 breaths/min and tidal volume of 12 ml/kg. Ten minutes prior to carotid artery clamping (right unilateral internal carotid artery) which was performed after heparinisation, pharmacologic mitigation was done for cerebral protection with a total dose of thiopentone 50 mg (2.5%), 8 mg dexamethasone, 100 mg hydrocortisone and 15 ml (7.5% w/v) sodium bicarbonate as i/v bolus and 250 ml dextran 40 (10% w/v) at 40 ml/hour as i/v drip. Mean values of arterial pressure and heart rate were 94±16 mmHg and 88±11 min-1 respectively, over the entire intra operative period. A moderate alkalosis which occurred in all animals under anaesthesia was postulated to supplement cerebral protection and was corrected by reducing the respiratory rate and tidal volume to 10 breaths/min and 10ml/kg respectively. Significant variation in pH (p<0.05) was observed at 90, 120, 150 and 180 minutes after induction of anaesthesia. Significant variation in MAP (p<0.05) was observed at 180 minutes after premedication, which was related to alkalosis and resultant hypokalemia and was effectively corrected with 31±7 meq potassium chloride (40 meq in 500 ml ringer lactate). The total procedure lasted 126±18 minutes and total unilateral right carotid arterial clamping time was 36.7±6.5 minutes. 28±5 minutes after spontaneous respiration, the animals were extubated and moved to the postoperative cage. Three doses of nadroparine 3800 IU s/c at 12 hour intervals were given postoperatively. All animals were free from any neurological deficits, which showed the effectiveness of the perioperative protocol encompassing the cerebral protective medication.
Introduction
Occlusion of major blood flow to the brain in both animals and humans reduces blood pressure (BP) in arteries distal to the point of occlusion (Freeman et al., 1994). Sheep under ischemia and cerebral circulatory arrest without cerebral protection presented behavioral and histological impairment (Crittenden et al., 1991), and neurological impairment has been recorded in dogs (Baumgartner et al., 1999), pigs (Midulla et al., 1994) and mice (Smith et al., 1984).
General anesthesia which maintains systolic arterial blood pressure within 20-30 mmHg of the baseline is recommended for cerebral protection in carotid endarterectomy (Wilson and Hobson II, 2004) in humans. Various regimens have been reported in animals which include hypothermic circulatory arrest (HCA), alpha-stat management where blood gases are regulated to create an alkaline pH during hypothermia, pH-stat management by incorporating more CO2 for acidification promoting cerebral vasodilatation resulting in “luxury perfusion”, topical cooling of brain, retrograde cerebral perfusion and pharmacologic strategies to mitigate cerebral injury (Juvonen et al., 2000).
Several combinations of pharmacological agents, topical cooling, alpha-stat and pH-stat managements with HCA have been tried in animals for cerebral protection during deprived blood supply to the brain with a major limitation that it does not address procedures where systemic hypothermia can not be instigated. The present study involved right unilateral internal carotid artery clamping, arterectomy and prosthesis without a cardiopulmonary bypass where HCA was not possible and for cerebral protection, balanced anesthesia and pharmacological mitigation were the only measures. A simple protocol encompassing cerebral protection without systemic hypothermia, aiding in a safe, smooth and fast recovery of sheep used for the preclinical evaluation of a prosthetic vascular graft in the carotid artery, is presented in this study.
This work focuses on anesthesia, preventive steps to be followed during an easy but effective monitoring while performing any such experimentation which deprives blood flow to the brain, and the procedure for cerebral protection. This addresses a wider platform of animal experimentation in the area of cerebral protection as well as anesthesia.
Materials and methods
The study group comprised ten adult male Ramnad white sheep weighing 33.8 ± 3.2 Kg housed under conventional environmental conditions with 12: 12 L: D lighting. Care and management of animals were in accordance with the recommendations of the Committee for the Purpose of Control and Supervision of Experiments in Animals (CPCSEA) Guidelines, Ministry of Environment and Forests, Government of India. Experiments were done after the review of Institutional Animal Ethics committee (IAEC) in the Division of In vivo Models and Testing, Sree Chitra Tirunal Institute for Medical Sciences and Technology (SCTIMST), Bio Medical Technology Wing (BMT Wing), Thiruvananthapuram, Kerala, India. The colony was outbred, open colony with animals procured from breeders located at Tirunelveli, Tamil Nadu, India. The animals were let loose daily during day time for five hours in a fenced run with watering pans and shaded areas to provide shelter in adverse climatic conditions. The animals were fed daily with a compounded ration and paddy straw to meet digestible crude protein and energy requirements with ad libitum drinking water.
Pre-conditioning
During the forty five days quarantine period, animals were dewormed
with 10 mg/kg albendazole orally and a clinical and hematological
examination was done prior to selection for experimentation.
Anticoagulant therapy was done with aspirin 150 mg (Ecospirin 150, USV
Ltd., Mumbai, India) and clopidogrel 75 mg (Clopivas USP, Cipla,
India) once daily orally, from five days prior to surgery till the end
of the study period and simultaneously, the animals were deprived of
green grass. Animals were fasted for 24 hours and water was withdrawn
with oral administration of one gram tetracycline, 12 hours prior to
procedure.
Anesthetic Premedication
Atropine sulphate (Tropine, Neon Lab, Thane, India) was given at
0.1mg/kg; half of the calculated dose was given intramuscularly (i/m)
and the remainder sub-cutaneously (s/c). Xylazine hydrochloride
(Xylaxin, Indian Immunologicals, Hyderabad, India) 0.75 mg/kg i/m was
given. After losing the righting reflex, the animals were transferred
to the surgical table. Keeping the heart rate (HR) and BP as
reference, normal saline (NS) infusion at 5 ml/kg/hour was given
through the cannulated jugular vein.
Induction of Anesthesia
The animals received 2 mg/kg ketamine hydrochloride (Aneket, Neon
Laboratories Limited, Thane, India), 5 mg/kg thiopentone sodium (2.5%)
(Thiosol sodium, Neon Laboratories Limited, Thane, India) and 0.02
mg/kg pancuronium bromide (Neocuron, Neon Laboratories Limited, Thane,
India) intravenously (i/v). With the help of a lubricated (Lignocaine
gel 2%) custom made stainless steel laryngoscope (26mm long), a 10 mm
endotracheal tube (SIMS Portex Ltd., Hythe, Kent, UK) was introduced
and secured in place along with the stomach tube (Quarter inch
diameter PVC tube of length 2 meter) by tying to the mouth gag. With a
preset tidal volume of 12 ml/kg, rate of 12 breaths/ minute and 1:1
Oxygen: Nitrous oxide ratio, positive pressure ventilation
(Datex-Ohmeda 7000, Madison, WI, USA) was initiated. Periodic suction
was applied to the stomach tube for draining the stomach contents
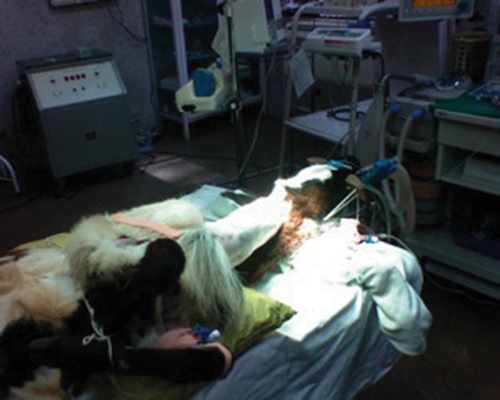
using an electric suction pump. The picture of total surgical set up
and animal positioning is given in Figure 1.
Maintenance of anesthesia
Using an i/v flow regulation extension set (Medireg, Eastern medikit,
Gurgaon, India), 500 mg ketamine hydrochloride mixed in 250 ml of
dextrose normal saline was given at 5 mg/kg/hour. Pancuronium bromide
0.01 mg/kg i/v (half the initial dose) was repeated at every 45
minutes. A total dose of 325 ± 16 mg ketamine and 0.71 ± 0.06 mg
pancuronium was used in animals during the maintenance of anesthesia.
Pharmacologic Mitigation
During the dissection, 1 to 2 ml lignocaine 2% solution was instilled
over the carotid sheath to avoid vagal stimulation. Heparin (Nuparin,
Troikaa, Gujarat, India) 1.5 mg/kg i/v was given ten minutes prior to
carotid clamping, and a total dose of 50 mg thiopentone sodium (2.5%),
8 mg dexamethasone (Dexasone, Cadila, Ahmedabad, India), 100 mg
hydrocortisone (hydrocortisone, Troikaa, Gujarat, India) and 15 ml
sodium bicarbonate (7.5%w/v) (Sodium bicarbonate BP, Troikaa, Gujarat,
India) was given as an i/v bolus. Dextran 40 (10% w/v) (Microspan-40,
Claris Life Sciences Limited, Ahmedabad, India) 250 ml at 40 ml/hour
was administered as i/v infusion.
Monitoring of Clinical parameters
Electrocardiogram (E.C.G) (3-Lead system), heart rate (HR), SpO2, and
body temperature were monitored intraoperatively using a
plethismography monitor (Passport Datascope Corporation, Paramus,
USA). Auricular artery was cannulated and mean arterial pressure (MAP)
was monitored using an invasive blood pressure (IBP) machine
(Hewlett-Packard GmbH, Germany). Anaerobically, 0.5 ml of arterial
blood was obtained from the same site at 30 minute intervals for blood
gas analysis (ABL-5, Radiometer, Copenhagen, Medical A/S, Denmark).
Complications and Correction
Ectopic ventricular beats observed after anesthetic premedication were
treated with 20 mg Lignocaine i/v (Lox 2% injection, Neon Laboratories
Limited, Thane, India). Flattening of T- wave and hypotension observed
in hypokalemia was treated with 31 ± 7 mEq potassium chloride
(Potassium hydrochloride injection USP, Hindustan pharmaceuticals,
Barauni, India) after mixing 40 mEq in 500 ml ringer lactate solution
as slow i/v whilst closely watching the ECG. Cerebral oedema was
treated with 1 g/kg mannitol i/v (20%w/v) (20M, Claris Life Sciences
Limited, Ahmedabad, India). Correction of alkalosis was done after the
completion of grafting by reducing the respiratory rate and tidal
volume to 10 breaths / minute and 10 ml/kg respectively.
Muscle relaxant reversal and Recovery phase
At the time of skin closure, muscle relaxation was reversed with a
mixture of 0.05 mg/kg atropine sulphate and 0.08 mg/kg neostigmine
methyl sulphate (Neocuron, Neon Laboratories Limited, Thane, India)
i/v. The animals were immediately brought to sternal recumbency and
rewarming was initiated. When spontaneous respiration became smooth
and regular, the animals were weaned from the ventilator. After
removing endotracheal and stomach tubes, along with SpO2, temperature
and E.C.G probes, the animals were transferred to a warmer floor and
oxygen delivery was continued with a custom made facemask. The animals
were then moved to a post-operative cage as soon as the righting
reflexes returned and they became fully responsive to external
stimuli.
Post-operative care
For the first week post operatively, animals were housed with another
juvenile male or female to reduce the stress when housed individually
and with other experimental animals afterwards. Anticoagulant therapy
was continued with three doses of nadroparine calcium (Fraxiparine,
Glaxosmithkline, Mumbai, India) 3800 IU s/c at 12 hour intervals
postoperatively. Postoperative antibiotic and analgesic therapy was
given with 1g streptomycin, 600,000 IU procaine penicillin and 200,000
IU of penicillin G sodium (Dicrysticin-DS) and paracetamol (10 mg/kg)
i/m twice daily for five days. Indirect assessment of the central
nervous system was done by monitoring the state of consciousness, body
temperature, patterns of breathing, menace reflex, feeding and
drinking and patency of the arterial graft was monitored using an
ultrasound doppler machine (Sonydop, Electronic Engineering
Corporation, India) daily for the first week postoperatively.
Angiographic patency was assessed in all the animals at the end of the
trial using a digital fluoroscopic C-arm (Power Mobil, Siemens,
Germany).
Analysis of Data
Data are expressed as mean ± standard deviation (SD). Paired,
two-tailed Student’s t-test was applied to compare MAP, HR and
pH between various time points intraoperatively within groups. A value
of P<0.05 was considered as statistically significant.
Results
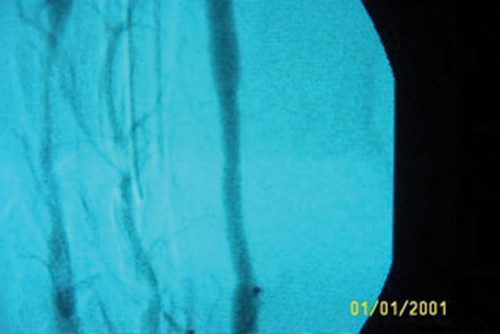 All the animals were free from any neurological deficits and could
complete their designated period of implantation without any
complications. This indicated that the perioperative protocol
encompassing the cerebral protective medication was effective when
performing a vascular graft in sheep carotid artery. Doppler analysis
revealed that there was no carotid obliteration during the first week
postoperatively. The angiogram of one animal at end of the study
period is shown in Figure 2. This demonstrates partial occlusion at
anastamosis of the test graft.
All the animals were free from any neurological deficits and could
complete their designated period of implantation without any
complications. This indicated that the perioperative protocol
encompassing the cerebral protective medication was effective when
performing a vascular graft in sheep carotid artery. Doppler analysis
revealed that there was no carotid obliteration during the first week
postoperatively. The angiogram of one animal at end of the study
period is shown in Figure 2. This demonstrates partial occlusion at
anastamosis of the test graft.
There existed a practical difficulty in obtaining MAP, HR and blood gas from the animals prior to premedication. The intra-operative MAP, HR and pH was compared with the initial values obtained after premedication. Mean values of MAP and HR were 94±16 mmHg and 88±11 min -1 respectively, over the entire intra-operative period. A moderate alkalosis was recorded in all the animals under anaesthesia, with alterations in arterial blood pH, pO2 and pCO2 values (7.48± 0.06, 180± 36 mmHg and 31± 5.3 mmHg respectively). Significant variation in pH (p<0.05) was observed at 90, 120, 150 and 180 minutes after induction of anaesthesia (Table II).
Table I. Physiological parameters monitored during anesthesia and recovery phase (Mean±SD)
| Time in minutes after premedication→ | 30 | 60 | 90 | 120 | 150 | 180 | 210 |
| Heart Rate (beats/min) | 90±14 | 87±21 | 85±5 | 92±4 | 88±10 | 86±8 | 88±4 |
| Invasive Blood Pressure | |||||||
| (MAP in mm of Hg) | 107±14 | 104±11 | 96±21 | 98±18 | 90±13 | 81±8* | 92±7 |
| Body Temperature (oC) | 38.0±0.4 | 37.7±0.6 | 36.7±0.5 | 36.4±0.4 | 36.9±0.7 | 37.1±0.5 | 38.2±0.2 |
| SpO2 (%) | 97±2 | 94±5 | 96±4 | 93±6 | 84±8 | 92±4 | 92±4 |
(*Significant difference when compared with initial value p<0.05)
Table II. Blood gas values obtained periodically intra-operatively for analysis and correction (Mean±SD)
| Time in Minutes after premedication→ | 60 | 90 | 120 | 150 | 180 | 210 |
| pH | 7.43±0.05 | 7.52±0.08* | 7.52±0.06* | 7.49±0.05* | 7.49±0.05* | 7.41±0.02 |
| pCo2 (mm of Hg) | 32±3 | 28±4 | 30±4 | 32±5 | 32±5 | 28±1 |
| pO2 (mm of Hg) | 108.4±8.2 | 168.6±21.5 | 179.2±23.7 | 165.4±7.5 | 122.4±9.4 | 106±6.2 |
| HCO3– (mEq) | 20.6±0.57 | 25±0.82 | 24.5±0.12 | 23±1.46 | 23±0.32 | 21.1±2.82 |
| Acid Base Excess (mmol/L) | –3±1 | 0 | 2±1 | 0 | 0 | 2±4 |
| K+ (mEq) | 3.2±0.06 | - | - | - | 2.4±0.12 | 3.4±0.09 |
(*Significant changes in pH when compared with initial value p<0.05)
Ectopic ventricular beats observed in one subject after 22 minutes of anesthetic premedication was effectively treated. Hypokalemia with hypotension observed in five animals, among which one showed T-wave flattening after completion of the skin suturing, and cerebral oedema observed in four animals was effectively corrected. Significant variation in MAP (p<0.05) was observed at 180 minutes after premedication (Table I), which was related to alkalosis and resultant hypokalemia.
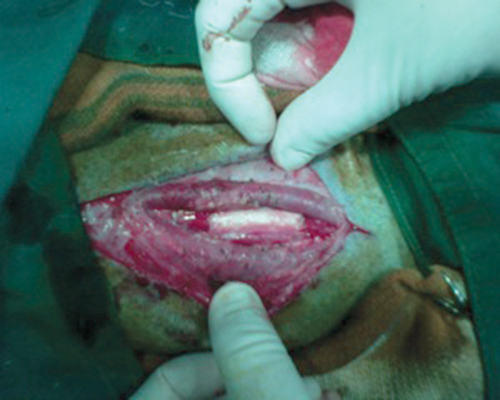 The total procedure took 126±18 minutes from pre-anesthetic
medication to the last skin suture. The carotid artery after resection
and prosthetic graft anastamosis in situ is shown in Figure
3. Total unilateral right carotid arterial clamping time was 36.7±6.5
minutes. After muscle relaxant reversal, pedal reflex was regained in
34 ±6 minutes and in 65±11 minutes spontaneous respiration was
restored. In 28±5 minutes after spontaneous respiration, the animals
were extubated and shifted to their respective postoperative cages.
The total procedure took 126±18 minutes from pre-anesthetic
medication to the last skin suture. The carotid artery after resection
and prosthetic graft anastamosis in situ is shown in Figure
3. Total unilateral right carotid arterial clamping time was 36.7±6.5
minutes. After muscle relaxant reversal, pedal reflex was regained in
34 ±6 minutes and in 65±11 minutes spontaneous respiration was
restored. In 28±5 minutes after spontaneous respiration, the animals
were extubated and shifted to their respective postoperative cages.
Discussion
All the animals were free from any neurological deficits and could complete their designated period of implantation without any complications. This indicated that the perioperative protocol encompassing the cerebral protective medication was effective when performing a vascular graft in sheep carotid artery. Doppler analysis revealed that there was no carotid obliteration during the first week postoperatively. The angiogram of one animal at end of the study period is shown in Figure 2. This demonstrates partial occlusion at anastamosis of the test graft.
There existed a practical difficulty in obtaining MAP, HR and blood gas from the animals prior to premedication. The intra-operative MAP, HR and pH was compared with the initial values obtained after premedication. Mean values of MAP and HR were 94±16 mmHg and 88±11 min -1 respectively, over the entire intra-operative period. A moderate alkalosis was recorded in all the animals under anaesthesia, with alterations in arterial blood pH, pO2 and pCO2 values (7.48± 0.06, 180± 36 mmHg and 31± 5.3 mmHg respectively). Significant variation in pH (p<0.05) was observed at 90, 120, 150 and 180 minutes after induction of anaesthesia (Table II).
Ectopic ventricular beats observed in one subject after 22 minutes of anesthetic premedication was effectively treated. Hypokalemia with hypotension observed in five animals, among which one showed T-wave flattening after completion of the skin suturing, and cerebral oedema observed in four animals was effectively corrected. Significant variation in MAP (p<0.05) was observed at 180 minutes after premedication (Table I), which was related to alkalosis and resultant hypokalemia.
The total procedure took 126±18 minutes from pre-anesthetic medication
to the last skin suture. The carotid artery after resection and
prosthetic graft anastamosis in situ is shown in Figure 3.
Total unilateral right carotid arterial clamping time was 36.7±6.5
minutes. After muscle relaxant reversal, pedal reflex was regained in
34 ±6 minutes and in 65±11 minutes spontaneous respiration was
restored. In 28±5 minutes after spontaneous respiration, the animals
were extubated and shifted to their respective postoperative cages.
Discussion
The Institutional Animal Ethics Committee constituted as per CPCSEA
norms of Ministry of Environments and Forests, Government of India as
well as other animal research guidelines states that ill effects
expected due to well documented protocols and procedures as well as
treatments shall be avoided if it is likely to cause an adverse effect
in experimental animals. Since the neurological adverse effects in
sheep due to circulatory loss are well documented, for the welfare of
laboratory animals, we have avoided using controls in this study.
Further, many citations are made showing adverse cerebral events in
laboratory animals undergoing surgeries that deprive blood flow to
cerebrum.
Balanced anaesthesia (Whelan and Flecknell, 1992) was selected which could provide minimum variations in cardiovascular functions and the dosage of each component was adjusted to give this result.
Ketamine is a well known nociceptive agent (Johnson et al., 1999) and its combinations were found effective (Ewing, 1990) in small ruminants. Sympatho-adrenal activation by ketamine is well documented, and increased cardiac output, raised vasomotor tone and tachycardia is attributable to this property (Brander et al., 1991). These advantages of ketamine made it the drug of choice in this study. Further, only 2 mg/kg was used for induction and 5 mg/kg/hour for maintenance and at these dosages, tachycardia and negative inotropic action (Brander et al., 1991) which are the direct effects of this drug on the heart and blood vessels were not present. Impairment of cardiovascular functions was reported for halothane (Taylor, 1998). Decreased cardiac output (Lin et al., 1997) and significant alterations in heart rate by propofol (Runciman et al., 1990) and hypotension by isofluorane (Taylor, 1990) anesthesia are salient features in animals.
The dose of thiopentone sodium for induction was reduced by using pancuronium and ketamine to prevent hypotension (Taylor, 1999) reduced cerebral blood flow (Upton and Ludbrook, 1999) and decreased cardiac output and stroke volume (Hall et al., 2000); this combination gave adequate muscle relaxation and apnoea for smooth endotracheal intubation. The dosage scheme of atropine in the present study was different to the dose and route mentioned by Holmberg and Olsen (1987), but was effective in counteracting the cardio-suppressive action of xylazine and producing prolonged anti-sialogogic effects against xylazine and ketamine. In addition xylazine at this dose avoided adverse effects on the cardiovascular system (Hall et al., 2000) and aided in smooth recovery without any episodes of excitation as reported in ketamine anesthesia (Brander et al., 1991). One third of the dose of pancuronium prescribed for simple stomached animals (Brander et al., 1991) was used in sheep to avoid circulatory side effects by inhibiting neuronal reuptake of noradrenalin. The dosage used during this work for muscle relaxant reversal differed from the dose mentioned by Brander et al. (1991) but was found to be very effective.
Thiopentone can be used as cerebral protective medication in procedures depriving blood flow to the brain (Juvonen, 2000). Siegman et al. (1992) used a bolus of sodium thiopental (40 mg/kg) during bypass at 37 °C followed by an infusion of 3.3 mg/kg/min until hypothermic arrest in sheep. Hypotension is a major draw back associated with the use of thiopentone during cerebral protection (Juvonen, 2000). In the present study, thiopentone was used effectively without any adverse effects on cardiovascular function and was accompanied with corticosteroids and dextran 40 to avoid hypotension or shock. Sodium bicarbonate helps in maintaining an alkaline pH which is advantageous for cerebral protection.
For cerebral protection, blood gases are regulated to maintain an alkaline pH so that cerebral autoregulation is well preserved with diminishing cerebral oxygen requirements (Juvonen et al., 2000). In the present study, even with a lower tidal volume compared with 15 ml/kg in calves (Wilson et al., 2000), alkalosis was present intraoperatively. In a similar study of carotid artery translocation in goats by Carroll et al. (1998), results on HR, MAP and body temperature were comparable with the present study except that they reported acidosis. Our findings were in contradiction to the previously observed respiratory acidosis in Ketamine and Tiletamine combinations (Howard et al., 1990) and the finding of Green (1979) that pre-operative fasting may produce metabolic acidosis and hypoglycemia. Alkalosis which was advantageous for cerebral protection intraoperatively was controlled by reduced ventilation parameters in the recovery phase. Animals were transferred from lateral to sternal recumbency in the recovery phase which also aided in return of blood pH to normal levels as previously reported by Fujimoto and Lenehan (1985) and Lin et al. (1993).
Hypokalemia and associated hypotension (Muir et al., 1990), resulting from respiratory alkalosis as reported by Hassan et al. (1979), was effectively treated with 40mEq potassium chloride i/v which was in contrast to the higher dosage of 80 mEq given by Holmberg and Olsen (1987). T-wave flattening was attributable to hypokalemia. Even though ketamine has antiarrhythmic properties (Brander et al., 1991), a case of ventricular ectopic beats was effectively treated with lidocaine without convulsions as reported in cats and dogs (Gillis et al., 1973).
Even after a longer duration of fasting, Wilson et al. (2000) reported occasional gastric tube blockage with feed material which stopped draining and resulted in bloat in calves. No perioperative complications were observed in the present study which featured 24 hour fasting, tetracycline administration, water withdrawal, anticoagulation combined with supplementation of straw instead of green grass to avoid the clotting factor vitamin K and reducing stress by providing a juvenile companion.
In human patients, carotid clamping induced neurological and cardiovascular adverse effects, loss of consciousness, and confusion (Callow, 2004 and Robicsek, 1982). However, the evaluation of cerebral function can not guarantee that no significant changes in intellectual functions have occurred in animals (Juvonen et al., 2000). Since most of the quasi physiologic measuring of cerebral perfusion and oxygenation are unreliable and cumbersome, (Callow, 2004) indirect assessment of the CNS was done by close observation of the general pattern of behavior specific to the species. The state of consciousness, patterns of breathing, reflex/reactivity and size of pupils, occular movements, skeletal musculomotor responses and reflexes were observed to indicate the normal neurological state of the animal. Proprioceptive and motor system evaluations were made by observation of smooth gait, stance, normal positioning of the limbs with normal strength in all the animals as suggested by Fenner (2000). These together with noninvasive Doppler study and final angiogram gave an estimate of cerebral perfusion and adequacy of the protocol.
This (Fenner, 2000) is a well established and documented method for clinically assessing the neurological symptoms of an animal. Boltze et al, 2008 used a scoring system, which measures several parameters to assess the neurological deficits of sheep, and in our work, we have assessed clinically the same parameters but as described by Fenner (2000). State of activity, torticollis, carpus and fetlock position in partial flexion, ataxia, circling, right hemistanding reaction, right hopping reaction and wheel barrowing were the terms used for analysis of sheep neurological deficits by Boltze et al 2008.
In our work, we used a clinical assessment of neurological symptoms as described by Fenner (2000) and assessed proprioception with motor system assessments which covered torticollis, ataxia, circling and right hemistanding. Gait analysis with normal positioning of limbs was done to detect if there were adverse effects on fetlock or carpal positioning. This is a well established system of clinical assessment which was useful in our work as well.
Selection of an appropriate regime for anaesthesia, anticoagulation, maintaining BP and HR within a normal range throughout the study and pharmacologic mitigation during the clamping procedure with perioperative management resulted in a successful study in the sheep model. Alkaline pH was useful for the procedure. Meticulous operative technique, removal of all debris, copious heparinised flushing and retrograde bleeding of internal carotid artery to remove the particulate debris eliminated any remaining chances for cranial embolisation and aided in an eventless recovery in sheep.
Acknowledgements
We are grateful to Dr. K. Mohandas, Director and Dr. G.S.
Bhuvaneshwar, Head of Biomedical Technology Wing, Sree Chitra Tirunal
Institute for Medical Sciences and Technology, Thiruvananthapuram,
Poojappura, Kerala, India for supporting this work.
References
-
Baumgartner WA, PL Walinsky, JD Salazar, EE Tseng, MV Brock, JR
Doty, JM Redmond, ME Blue, MA Goldsborough, JC Troncoso & MV
Johnston:Assessing the impact of cerebral injury after cardiac surgery:
Will determining the mechanism reduce this injury?. Ann. Thorac.
Surg. 1999, 67, 1871-1873.
-
Boltze J, FAnnette, N Björn, W Daniela, H Anke, CM Boltze,
YD Antje, G Axel, R Anne, H Wolfgang , DG Kathrin, B Henryk,
E Frank & G Uwe: Permanent middle cerebral artery occlusion in sheep: a novel
large animal model of focal cerebral ischemia. J. Cereb. Blood.
Flow. Metab.2008, 28, 1951–1964.
-
Brander GC, DM Pugh, RJ Bywater & WL
Jenkins:Veterinary applied pharmacology and therapeutics. 5th edn. London: Bailliere Tindall, 1991.
-
Callow A: Cerebral protection during carotid artery
surgery. In: Vascular surgery- Principles and practice3rd edn.
(Hobson RW II, SE Wilson & FJ Veith eds.) New York: Marcel
Dekker Inc. 2004.
-
Carroll GL, RN Hooper, MR Slater, SM Hartsfield & NS
Matthews: Detomidine butorphanol- propofol for carotid artery translocation
and castration or ovariectomy in goats. Vet. Surg. 1998,
27, 75-82.
-
Crittenden MD, CS Roberts, L Rosa, SK Vatsia, D
Katz, RE Clark & JA Swain:Brain protection during circulatory arrest. The Annals of Thoracic
surgery. 1991, 51, 942-947.
-
Ewing KK: Anesthesia techniques in sheep and goat. Vet.
Clin. North Am. Food Anim. Pract. 1990, 6:759-778.
-
Fenner WR: Diseases of the brain. In: Textbook of
Veterinary Internal Medicine Vol-I.5th edn (Ettinger SJ & EC
Feldman eds.) Philadelphia: Saunders, 2000.
-
Freeman DE, WJ Donawick & LV Klein: Effect of Ligation
on Internal Carotid Artery blood pressure in horses. Vet. Surg.
1994, 23, 250-256.
-
Fujimoto JL & TM Lenehan: The influence of body
position on the blood gas and acid base status of halothane
anesthetized sheep. Veterinary Surgery. 1985, 14,
169-172.
-
Gillis RA, FH Levine, H Thibodeaux, A Raines & FG
Standaert: Comparison of Methyllidocaine and Lidocaine on Arrhythmias
Produced by Coronary Occlusion in the Dog. Circulation. 1973,
47, 697-703.
-
Green CJ: Laboratory Animal handbooks 8- Animal Anesthesia.
London: Laboratory Animals Ltd. 1979.
-
Hall LW, KW Clarke & CM Trim: Veterinary Anaesthesia
10th edn. Oxford: Elsevier Health Sciences. 2000.
-
Hassan H, J Gjessing & PJ Tomlin: Hypercapnia and
hyperkalemia. Anesthesia. 1979, 34, 897-899.
-
Holmberg DL & DB Olsen: Anesthesia and Cardiopulmonary
bypass technique in Calves and sheep. Vet. Surg. 1987, 16,
463-465.
-
Howard BW, MS Lagutchik, J Januszkiewicz & DG Martin:
The cardiovascular response of sheep to tiletamine-zolazepam and
butorphanol tartrate anaesthesia. Vet. Surg. 1990,
19, 461-467.
-
Johnson CB, M Bloomfield & PM Taylor: Effects of
ketamine on equine encephalogram during anaesthesia with halothane
in Oxygen. Vet. Surg. 1999, 28, 380-385.
Juvonen T, V Anttila & MA Ergin: Brain protection during aortic arch surgery. Scand. Cardiovasc. J. 2000, 34, 106-115.
-
Lin HC, RC Purohit & TA Powe: Anesthesia in sheep with
propofol or with xylazine ketamine followed by halothane. Vet.
Surg.1997, 26, 247-25.
-
Lin HC, JW Tyler, EG Welles, JS Spano, JC Thurmon & DF
Wolfe: Effects of anesthesia induced and maintained by
continuous intravenous administration of guaifenesin, Ketamine and
xylazine in spontaneously breathing sheep. Am. J. Vet. Res. 1993,
54, 1913-1916.
-
Midulla PS, A Gandsas, AM Sadeghi, CK Mezrow, ME Yerlioglu, W
Wang, D Wolfe, MA Ergin & RB Griepp: Comparison of retrograde cerebral perfusion to antegrade cerebral
perfusion and hypothermic circulatory arrest in a chronic porcine
model. J. Card. Surg. 1994, 9, 560-575.
-
Muir WW III, AE Wagner & C Buchanan: Effects of Acute
hyperventilation on serum potassium in dogs. Vet. Surg. 1990,
19, 83-87.
-
Robicsek F: Post operative monitoring- Role of Intensive
care units. In: Vascular emergencies. (Haimovici H eds.) New York:
Appleton- Century-crofts. 1982.
-
Runciman WB, LE Mather & DG Selby: Cardiovascular
effects of propofol and of thiopentone anaesthesia in the sheep. Br.
J. Anaesth. 1990, 65, 353-359.
-
Siegman MG, Anderson RV, Balaban RS, Ceckler TL, Clark RE &
Swain JA: Barbiturates impair cerebral metabolism during hypothermic
circulatory arrest. Ann. Thorac. Surg. 1992, 54,
1131-1136.
-
Smith ML, RN Auer & BK Siesjo: The density and
distribution of ischemic brain injury in the rat following 2–10 min
of forebrain ischemia. Acta Neuropathol. 1984, 64,
319-332.
-
Taylor PM: The stress response to anaesthesia in ponies:
Barbiturate Anaesthesia. Equine Vet. J.1990, 22,
307-312.
-
Taylor PM: Endocrine and Metabolic responses to halothane
and pentobarbitone anaesthesia in Sheep. J. Vet. Anaesth. 1998,
25, 24-30.
-
Taylor PM: Effects of hypertonic saline infusion on the
adrenocortical response to thiopental-halothane anaesthesia in sheep
after premedication with Acepromazine. Vet. Surg. 1999, 28, 77-82.
-
Upton RN & GL Ludbrook: A model of the kinetics and
dynamics of induction of anaesthesia in sheep: variable
estimation for thiopental and comparison with propofol. Brit. J.
Anaesth.1999, 82, 890-899.
-
Whelan G & PA Flecknell: The assessment of depth of
anaesthesia in animals and man. Lab. Anim. 1992,
26, 153-162.
-
Wilson SE & RW Hobson II: Extracranial carotid artery
occlusive disease. In: Vascular surgery- Principles and practice.3rd
edn. (Hobson RW II, Wilson SE & Veith FJ eds.) New York: Marcel
Dekker Inc. 2004.
- Wilson DV, A Kantrowitz, J Pacholewicz: Perioperative management of calves undergoing implantation of a left ventricular assist device. Vet. Surg. 2000, 29, 106-118.
