Quantitative Analysis of Heroin and its Metabolites in vivo
by Farong Yu*, Xiuzhen Lian, Hongmei Zhang, Mingren Xie, Lu Zhang
Key Laboratory of Evidence of Science and Technology Research and
Application, Gansu Institute of Political Science and Law, Lanzhou
730070 China
 Correspondence: Farong Yu
Correspondence: Farong Yu
Key Laboratory of Evidence of Science and Technology Research and
Application,
Gansu Institute of Political Science and Law, Lanzhou 730070, China
Tel (86) 130 8876 9779
Fax (86) 0931 2600596
E-mail fyu@ufl.edu
Summary
This study investigated heroin and its metabolites in vivo in the rat by using a high-performance liquid chromatography-tandem mass spectrometry (GC-MS) method. After intramuscular administrationof heroin (10 g/kg body weight) or morphine hydrochloride (20 mg/kg body weight) for 14 consecutive days, the heroin metabolites 6-AM (6-monoacetylmorphine) and morphine were detected in hair and urine samples.. In both the morphine and heroin groups, urine samples contained much higher levels of morphine than 6-AM, while hair samples contained equivalent levels of morphine and 6-AM. The presence of 6-AM in the morphine group might be related to the acetylation of morphine into heroin by the acetyl-transferring enzyme in liver, a conversion process that was confirmed in laboratory experiments but has not previously been described in vivo in mammalian species including humans In addition, our study also found that the formation of morphine not only can result from the metabolism of heroin but can also be generated by taking morphine hydrochloride.
Introduction
Heroin (diacetylmorphine) is a semi-synthetic opioid drug that was first synthesized in 1874. It is a highly addictive drug which is rapidly metabolized to 6-monoacetylmorphine (6-AM) and then to morphine (Roth et al., 2003), and is one of the most frequently abused opioid drugs. Heroin abuse or addiction presents a serious threat to human health and concerns for global public health (Guttinger et al., 2003; van den Brink et al., 2003).
Heroin has three major active metabolites, 3-monoacetylmorphine
(3-AM), 6-monoacetylmorphine (6-AM) and morphine, which can be
quantified in blood, plasma and urine to monitor for abuse, confirm a
diagnosis of poisoning and to assist in a medicolegal death
investigation (Rook et al., 2005). 6-AM is rapidly formed
from heroin in the body, and then is either metabolized into morphine
or excreted in urine (Bosch et al., 2007). As with other
opioids, heroin is used as both an analgesic and a recreational drug,
including the treatment for acute pain, such as in severe physical
trauma, myocardial infarction, post-surgical pain and chronic pain
(van den Brink et al., 1999; Dettmeyer et al., 2000; van den Brink
and Ree, 2003; van den Brink et al., 2003). High blood-brain permeability and effective delivery of morphine
to brain have been considered as explanations for the high potency of
heroin (Boix et al., 2011). Heroin-assisted treatment
significantly reduced the drug seeking behavior, and consequently led
to significant improvement of physical health, mental status and
social functioning of heroin dependent patients (van den Brink et al., 2003). However frequent and regular administration of heroin is
associated with tolerance and physical dependence, which may develop
into addiction.
Research using animals has contributed immensely to our understanding
of drug abuse and its consequences, prevention, and treatment. Animal
models of drug abuse and addiction can be useful tools for
understanding mechanisms of drug action. These models can provide
useful information on strategies for preventing and treating abuse and
addiction and for evaluating possible pharmacotherapies for safety and
efficacy before these therapies are tested in humans. However,
screening and confirmation for abuse of drugs and their metabolites
present many challenges for toxicological analysis (Aleksa et al., 2011).
In recent years, forensic toxicologists have been particularly concerned about distinguishing in drug testing between evidence of heroin abuse and the medical use of morphine or codeine and the consumption of poppy seeds in food. Hair and urine tests are common methods of detecting the presence of heroin by measuring metabolites. The determination of morphine in hair is a promising, convenient tool for identifying past, chronic heroin consumption (e.g., over 6 months) (Marigo et al., 1986; Nakahara et al., 1994; Paterson et al., 2011). The presence of drugs in urine provides only evidence of past exposure rather than evidence of a person being under the influence of a drug at a particular point in the past. However, presence of 6-AM in urine guarantees that heroin was in fact used as recently as within the last 24 hours (Nakahara et al., 1994; Paterson et al., 2011).
In the present study, a high-performance liquid chromatography-tandem mass spectrometry (GC-MS) method was used for the quantitative analyses of heroin and its major metabolites in Wistar rats addicted to heroin and morphine hydrochloride. Both 6-AM and morphine were measured and evaluated in hair and urine samples.
Materials and methods
Reagents and drugs
The reagents used in this study included Naloxone at 0.4 mg/ml
(Medical Technology Co., Ltd. Beijing), N-methyl-N-trimethylsilyl
trifluoroacetamide (MSTFA) (Sigma Corporation of America, USA),
acetonitrile (BDH Merck Ltd., UK), and phosphate buffer (pH 8.4),
sodium sulfide, soap powder and starch, which were purchased from
local chemical companies in Lanzhou, China.
The drugs used included morphine hydrochloride and heroin (white powder, purity in 86.8%), both of which were obtained from the Lanzhou City Public Security Bureau, China. Qualitative validation of the optimized chromatographic conditions was completed in accordance with the guidelines published by the United Nations Office on Drugs and Crime (UNODC).
Experimental animals
A total of 60 adult male Wistar rats (animal certification number NO.
14-008) in the weight range of 200 - 220 g were obtained from the
Experimental Animal Center of the Gansu Medicine Institute, China, and
fed standard pellet diet and water ad libitum. Animals were
housed one per cage in a temperature-controlled room at 24±1°C and
maintained on a 12:12 h light-dark cycle. Each cage was filled with
padding materials. Prior to the experiments, all animals were
maintained for an acclimatization period of 7 days under the
laboratory conditions.
Sixty rats were randomly divided into 3 groups of 20 animals each. The dorsal hair of each animal was removed with a hair removal cream before experimental treatment. The thigh muscles of the rats were chosen as the sites to administer an intramuscular injection (im) of drugs. Animals in the control group were treated with 20 mg/kg body weight of normal saline bid (twice a day), im. Animals in the morphine and heroin groups were administered 20 mg/kg body weight of morphine hydrochloride and 10 g/kg body weight of heroin bid, im, respectively. All experiments and observations were done at the Key Laboratory of Science and Technology Research and Application of Gansu Province in the Gansu Institute of Political Science and Law, China.
Animal model of addiction
To develop an animal model of drug addition, the rats in the morphine
and heroin groups were administered daily with the same doses of
morphine hydrochloride and heroin as described above for 14
consecutive days. Following the final drug administration (day 14),
animals were given an intraperitoneal injection of 6.0 mg/kg body
weight of naloxone. When the treated animals presented some
withdrawal symptoms, including writhing, wet dog shaking, writhing,
jumping, grooming, teeth chattering, diarrhea and so on, the presence
of an animal model of drug addiction was confirmed.
Sample preparation
Hair samples (4 cm in length, weighing 100 to 200 mg), which had no
pre-treatment, were cut near the dorsal scalp of all experimental
animals. To remove any external contamination, all samples were
washed with 10 ml of ethanol, PBS buffer with 0.1% sodium dodecyl
sulfate (SDS) solution, and acetone. Samples were then cut into small
pieces (~0.5 mm) and incubated with 1 ml of 0.1 mol/L HCl in a water
bath at 45 0C for 18 h. The mixture was neutralized with 1 ml
phosphate buffer solution (pH 8.4) and extracted using 2 ml of a
solvent mixture of chloroform-isopropyl alcohol-heptane (50:17:33).
Urine samples were collected by using the metabolic cage for rats
provided by the Bejing Good Source Industrial Science and Technology
Co., Ltd, China.
Extraction was carried out by vortexing for 2 min, and then centrifuging at 750 g for 10 min at room temperature. The organic phases were transferred into glass conical tubes. The aqueous phases were re-extracted by the same mixture solvent. The final extraction was derivatized at 70 0C for 30 min by adding 50μl of the mixture of N-methyl-trifluoroacetamide:acetonitrile (1:1).
Urine samples were collected from animals with some withdrawal symptoms 1 h following the final drug injection (day 14). The collected samples were adjusted to pH 9.0 with 5% NaOH for further investigation. For each urine sample, two tubes were each filled with 2 ml adjusted urine solution. To both tubes were added 0.5 ml of boric acid (pH 9.2) and 2 ml of the mixed solvent of chloroform: isopropanol (9:1) and then the tubes were centrifuged at 750 g for 10 min at room temperature. The organic layer of each tube was transferred to a centrifuge tube that was dried with nitrogen at 50 0C. 50 μl of acetic acid and methanol were added to one tube for 6-AM analysis. To the other tube 50 μl of pyridine and 100 μl of acetic anhydride were added and derivatized at 60 0C for 30 min, evaporated to dryness, and then 50 μl of ethyl acetate was added for morphine analysis.
Levels of morphine and 6-AM in body hair and urine of addicted
rats
Hair and urine samples were analyzed by a HP 6890/5973 GC-MS (gas
chromatography-tandem mass spectrometry). The mass spectrometer was
operated in selective ion monitoring mode (SIM) and ions monitored
were at m/z 236, 414, 429 for morphine and at m/z 287, 340, 399 for
6-AM.
Statistical analysis
Statistical analysis was performed with the SPSS10.0 software package
for one-way ANOVA and measurements were presented as mean ± SD
(standard deviation). Student’s t-test was applied for
calculating the statistical significance of differences between
groups. The difference between groups at the p <0.05 level was
considered statistically significant.
Results
After being administered intramuscularly with heroin or morphine hydrochloride for 14 consecutive days, rats in both groups presented withdrawal symptoms which shows that animal models of addiction were successfully developed. Although both the heroin metabolites 6-AM and morphine were detected in body hair and urine samples, metabolite results for the morphine group were significantly different from results for the heroin group.
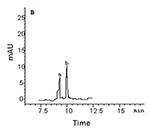
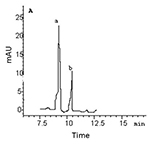
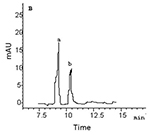
In hair samples, the levels of 6-AM and morphine in the morphine group were significantly higher than those in the heroin group, but the ANOVA (one way) showed no significant differences between 6-AM and morphine within each group (Table 1, Fig. 1). In urine samples, the levels of morphine (83-87% of the total concentration of the two metabolites) were significantly higher than those of 6-AM (13-17% of the total concentration of the two metabolites) in both morphine and heroin groups (Table 1, Fig. 2). Although the hair concentration of 6-AM was higher after morphine administration than that after heroin, injection of morphine resulted in a lower urine concentration of 6-AM compared to injection of heroin.
Table 1. Concentrations of morphine and 6-AM in the hair (ng/mg) and urine (µ/ml) samples of addicted rats (Mean ± SD, n = 20).
| Samples | Metabolites | Control group | Morphine group | Heroin group |
| Hair | Morphine | 0 | 4.66±1.1**▲▲ | 2.64±0.9** |
| 6-AM | 0 | 4.56±1.3**▲ | 2.88±0.6** | |
| Urine | Morphine | 0 | 32.3±0.6**▲▲ | 27.7±0.6** |
| 6-AM | 0 | 4.6±0.4**▲ | 5.2±0.2** |
**P<0.01 vs the control group;
▲▲P<0.01 vs the heroin group;
▲P<0.05 vs the heroin group.
|
Figure 1. GC-MS-SIM of the heroin metabolites from the hair samples of rats addicted to morphine (a) and heroin (b). A: morphine, B: 6-AM
Click images to enlarge |
|
|
Figure 2. GC-MS-SIM of the heroin metabolites from the urine samples of rats addicted to morphine (a) and heroin (b). A: morphine, B: 6-AM
Click images to enlarge |
Discussion
Animal models of drug abuse and addiction, as well as models for different consequences of drug abuse, can be useful tools for understanding mechanisms of drug action. The pharmacodynamic effects of heroin depend on the pharmacokinetic profile of heroin and its metabolites following different routes of administration. Urine, blood, and hair screening are the most common methods of detecting the use of heroin and other drugs. Experimental results based on the GC-MS analysis in this study indicated that the heroin metabolites 6-AM and morphine were detected from both urine and body hair samples of rats addicted to heroin or morphine hydrochloride, and the concentration levels of the two metabolites were significantly different between samples.
One of the most interesting results in this report is the presence of 6-AM in both hair and urine samples in the morphine group. The presence of 6-AM has been considered unique to the metabolism of heroin (Rook et al., 2006). However, in the present study, when animals were treated with morphine hydrochloride, 6-AM was detected in both the hair and urine samples (Figs. 1 and 2). Evidence indicates that the actions of heroin are due initially to heroin itself which subsequently could rapidly be hydrolyzed to 6-AM and finally metabolized to morphine in blood, liver, and kidney (Boerner, 1975; Skopp et al., 1977; Rook et al., 2006). The acetylation of morphine to yield heroin was confirmed in laboratory experiments (Bedford et al., 1987; Shults, 2009). The presence of 6-AM in the morphine group is apparently related to this conversion process. Morphine hydrochloride is metabolized mainly in liver and either reacted with glucuronic acid, demethylated or converted to heroin by the acetyl-transfer enzyme. The converted heroin is hydrolyzed to 6-AM, and then to morphine. However, this result has not previously been described in vivo in mammals (including humans) and more research on this conversion needs to be undertaken before the association between morphine hydrochloride and 6-AM is more clearly understood.
Heroin is rapidly absorbed following intranasal or intramuscular routes of administration and there are heroin peaks in serum in 3- 5 minutes in humans (Skopp et al., 1977), but the effects of heroin depend on the method of administration. When taken orally, heroin undergoes extensive first-pass metabolism via deacetylation, making it a prodrug for the systemic delivery of morphine (Sawynok, 1986). Compared to intravenous injection which provides the greatest intensity and most rapid onset of euphoria (7 to 8 seconds), intramuscular injection produces a relatively slow onset of euphoria (5 to 8 minutes) (Frank, 2000). In an intramuscular injection, drugs are delivered directly into a muscle and then circulated to body organs and tissues. The hydrolysis of heroin and 6-AM is catalyzed by different types of esterases that are abundantly present in blood and tissues (Salmon et al., 1999). Heroin blood levels in humans declined very rapidly after drug administration and became undetectable after 10-40 min (Rook et al., 2006), indicating that heroin is virtually fully converted into its metabolites before renal excretion. The high morphine levels in urine in the present study (Table 1 and Fig. 2) are likely to be caused by a high rate of heroin metabolism in blood, suggesting that the kidney is primarily involved in the excretion of morphine following heroin administration (Mo and Way, 1966; Elliott et al., 1971; van den Crugten et al., 1991).
Analysis of urine or blood specimens cannot determine the time of drug use with certainty. Hair drug analysis is proven to solve this problem and can accurately detect and measure drug molecules embedded in hair (Pragst and Balikova, 2006), especially 6-AM which can easily be hydrolyzed to morphine in blood.Hair is capable of recording medium to long-term or high dosage substance abuse. Morphine is a drug of abuse with the ability to down-regulate immune responsiveness that could have potentially serious consequences in both heroin addicts and in the clinical environment (Freier and Fuchs, 1994). The presence of 6-AM and morphine after injection of heroin are consistent with those of previous studies, but the observed 6-AM in the morphine group does not support the previous research (Table 1). The present study also found that the formation of morphine not only can result from the metabolism of heroin but can also be generated by taking morphine hydrochloride.
Conclusion
This study shows that, when rats were addicted to heroin or morphine hydrochloride, the heroin metabolites 6-AM and morphine were observed in both hair and urine samples. The high concentration of morphine in the urine samples (83-87% of the total concentration of the two metabolites) indicated that the administered heroin and morphine hydrochloride are virtually totally converted into their metabolites before renal excretion. The presence of 6-AM in both hair and urine samples of rats addicted to morphine hydrochloride could be related to the acetylation of morphine into heroin by the acetyl-transferring enzyme in liver. However, this result should be interpreted with caution and more research needs to be undertaken before this conversion process is more clearly understood.
Acknowledgements
This study was supported by the grants from the Natural Science Foundation of Gansu Province, Gansu Finance Education (2010: 148), Gansu Province, China, the Gansu University Academic Leaders Foundation (Ganjiaozi 2011: 23), and the Key Laboratory of Evidence of Science and Technology Research and Application (ZDSYS-Y17), Gansu Province, China,
References
-
Aleksa K, P Walasek, N Fulg, B Kappur, J Gareri & G Koren: Simultaneous detection of seventeen drugs of abuse and metabolites in hair using solid phase micro extraction (SPME) with GC/MS. Forensic Sci Int 2011.
-
Bedford KR, SL Nolan, R Onrust & JD Siegers: The
illicit preparation of morphine and heroin from pharmaceutical
products containing codeine: homebake laboratories in New Zealand.
Forensic Sci Int 1987, 34, 197-204.
-
Boerner U: The metabolism of morphine and heroin in man.
Drug Metab Rev 1975, 4, 39-73.
-
Boix F, JM Andersen & J Morland: Pharmacokinetic
modeling of subcutaneous heroin and its metabolites in blood and
brain of mice. Addict Biol 2011,
-
Bosch ME, AR Sanchez & Rojas: Morphine and its
metabolites: analytical methodologies for its determination. J Pharm
Biomed Anal 2007, 43, 799-815.
-
Dettmeyer R, G Stojanovski & B Madea: Pathogenesis of
heroin-associated glomerulonephritis: correction between the
inflammatory activity and renal deposits of immunoglobulin and
complement? Forensic Sci Int 2000, 113, 227-231.
-
Elliott HW, KD Parker, JA Wright & N Nomof: Actions and
metabolism of heroin administered by continuous intravenous infusion
to man. Clin Pharmacol Ther 1971, 12, 806-814.
-
Frank B: An overview of heroin trends in New York City:
past, present and future. Mount Sinai J Med 2000, 67,
304-346.
-
Freier DO & BA Fuchs: A mechanism of action for
morphine-induced immunosuppression: corticosterone mediates
morphine-induced suppression of natural killer cell activity. J
Pharmacol Exp Ther 1994, 270, 1127-1133.
-
Guttinger F, P Gschwend, B Schulte, J Rehm & A
Uchtenhagen: Evaluating long-term effects of heroin-assisted treatment: the
results of 6-year follow-up. Eur Addict Res 2003, 9,
73-79.
-
Marigo M, F Tagliaro, C Poiese, S Lafisca & C Neri:
Determination of morphine in the hair of heroin addicts by high
performance liquid chromatography with fluorimetric detection. J
Anal Toxicol 1986, 10, 158-161.
-
Mo BP & EL Way: An assessment of inhalation as a mode
of administration of heroin by addicts. J Pharmacol Exp Ther 1966,
154, 142-151.
-
Nakahara Y, R Kikura & K Takahashi: Hair analysis for
drugs of abuse VIII. Effective extraction and determination of
6-acetylmorphine and morphine in hair with trifluoroacetic
acid-methanol for the confirmation of retrospective heroin use by
gas chromatography-mass spectrometry. J Chromatogr B 1994,
657, 93-101.
-
Paterson S, S Lee & R Cordero: Analysis of hair after
contamination with blood containing 6-acetylmorphine and blood
containing morphine. Forensic Sci Int 2011, 210,
129-132.
-
Pragst F & MA Balikova: State of the art in hair
analysis for detection of drug and alcohol abuse. Clin Chim Acta
2006, 370, 17-49.
-
Rook EJ, MJX Hillebrand, H Rosing H, JM van den Ree & JH
Beijnen: The quantitative analysis of heroin, methadone, and their
metabolites and the simultaneous detection of cocaine, acetylcodeine
and their metabolites in human plasma by high-performance liquid
chromatography coupled with tandem mass spectrometry. J Chromatogr B
2005, 824, 213-221.
-
Rook EJ, ADR Huitema, W van den Brink, JM van den Ree & JH
Beijnen: Pharmacokinetics and pharmacokinetic variability of heroin and
its metabolites: review of the literature. Curr Clin Pharmacol 2006,
1, 109-118.
-
Roth SH, L Antonilli, F Semeraro, C Suriano, L Signore, P Nencini
& V Erspamer: High levels of morphine-6-glucuronide in street heroin addicts.
Psychopharmacology (Berl) 2003, 170, 200-204.
-
Salmon AY, Z Goren, Y Avissar & H Soreq: Human
erythrocyte but not brain acetylcholinesterase hydrolyses heroin to
morphine. Clin Exp Pharmacol Physiol 1999, 16, 596-600.
-
Sawynok J: The therapeutic use of heroin: a review of the
pharmacological literature. Can J Physiol Pharmaco 1986,
64, 1-6.
-
Shults TF: Medical Review Officer Handbook. 9th Ed.,
Quadrangle Research, 2009.
-
Skopp G, B Ganssmann, EJ Cone& R Aderjan: Plasma
concentrations of heroin and morphine-related metabolites after
intranasal and intramuscular administration. J Anal Toxicol 1977,
21, 105-111.
-
van Crugten JT, BC Sallustio, RL Nation & AA Somogyi:
Renal tubular transport of morphine, morphine-6-glucuronide, and
morphine-3-glucuronide in the isolated perfused rat kidney. Drug
Metab Dispos 1991, 19, 1087-1092.
-
van den Brink W, VM Hendriks & P Blanken: Medical
prescription of heroin to treatment resistant heroin addicts: two
randomised controlled trials. BMJ 2003, 32, 310-315.
-
van den Brink W, VM Hendriks & JM van Ree: Medical
prescription of heroin to chronic treatment resistant methadone
patients in the Netherlands. J Drug Issues 1999, 29,
587-608.
- van den Brink W & JM van Ree: Pharmacological treatments for heroin and cocaine addiction. Eur Neuropsychopharmaco 2003, 13, 476-487.