Recommendations for Urine and Urinary Bladder Collection in Chemical Carcinogenesis Assays with Rodents
by D. Talhada1▲, A. Andrade1▲, A.I. Faustino-Rocha1▲, C.I. Teixeira-Guedes1▲, J.H. Teixeira1▲, R. Arantes-Rodrigues1, C. Vasconcelos-Nóbrega2, R. Gil da Cost 3, P.A. Oliveira1, 4*
▲These authors contributed equally to this work
1Department of Veterinary Sciences, University of Trás-os-Montes and Alto Douro, UTAD, www.utad.pt, 5001-911, Vila Real, Portugal
2Agrarian School of Viseu, Polytechnic Institute of Viseu, Viseu, Portugal
3Laboratory for Process, Environmental and Energy Engineering, Faculty of Engineering, University of Porto, Porto, Portugal
4Centre for the Research and Technology of Agro-Environmental and Biological Sciences (CITAB), University of Trás-os-Montes and Alto Douro, UTAD, www.utad.pt, 5001-911, Vila Real, Portugal
 Correspondence: Paula A. Oliveira, PhD
Correspondence: Paula A. Oliveira, PhD
Department of Veterinary Sciences, CITAB, University of Trás-os-Montes and Alto Douro,
5001-911, Vila Real, Portugal
Tel +351259350651,
Fax +351259350480
E-mail pamo@utad.pt
Summary
This review describes the technical procedures to collect and process urine and urinary bladder samples, during and at the end of urinary bladder carcinogenesis assays with small rodents. The applications, advantages and disadvantages of each method are also mentioned and discussed.
Urinary bladder cancer
Urinary bladder cancer is the ninth most common malignant neoplasia and the thirteenth most frequent cause of death in humans worldwide. It is also the second most common cause of death in patients with malignant urogenital lesions (Jemal et al. 2011). Although transitional cell carcinoma is the most frequent histological type, adenocarcinomas, squamous cell carcinomas and sarcomas also occur (Kompier et al. 2010). The majority of cases (75-85%) are low-grade papillary carcinomas, with low invasive and metastatic potential. The others (15-25%) are high-grade invasive carcinomas that have their origin in flat lesions (hyperplasia, dysplasia and in situ carcinoma) (Cohen,2002; Kompier et al. 2010; Petraki & Sfikas,2008).
Several animal models have been used to study urothelial carcinogenesis, namely the dog (Canis lupus familiaris), rabbit (Oryctolagus cuniculus), guinea pig (Cavia porcellus), rat (Rattus norvegicus)and mouse (Mus musculus) (Oliveira et al. 2006). From the above described animals, Okajima, et al. (1981) considered that the dog is the ideal model to study human urinary bladder cancer, since dogs develop simultaneously both papillary and invasive carcinomas, just like human patients. However, dogs are unsuitable for experimental carcinogenesis studies, due to ethical and economical issues. Rabbits and guinea pigs have specific housing requirements that often limit their use. Small rodents such as rats and mice are easily housed and their biological characteristics are well-known (Oliveira et al. 2006). For these reasons, rats and mice have become the most commonly used animal models for the study of urinary bladder carcinogenesis (Arentsen et al. 2009). On the other hand, small laboratory rodents show no genetic predisposition to spontaneously develop urinary bladder cancer and researchers have to use chemical carcinogens, genetically modified animals or implanted urothelial carcinoma cell lines (xenograft and syngeneic models) (Clayson et al. 1995).
Urinary bladder chemical carcinogens
Various chemical carcinogens have been used to induce the development of tumours in the urinary bladder of laboratory animals. These carcinogenic agents may be administered subcutaneously, orally (drinking water, food or gavage) or by intravesical instillation.
The first compound described as an urinary bladder carcinogen was 2-acetylaminofluorene. However, this compound acts as a pluripotent agent with the ability to induce tumours in several other organs and, for this reason, it is little used in carcinogenesis experiments (Cohen,1998). The most effective compounds used to induce urinary bladder tumoursare N-[4-(5-nitro-2-furyl)-2-thiazolyl] formamide, N-methyl-N-nitrosourea and N-butyl-N-(4-hydroxybutyl) nitrosamine (Vasconcelos-Nóbrega et al. 2012). N-[4-(5-nitro-2-furyl)-2-thiazolyl] formamide is now little used due to laboratory and environmental safety concerns and also because it must be incorporated into the food for a long time (8 to 11 months) (Becci et al. 1981; Irving et al. 1984). N-methyl-N-nitrosourea may be incorporated into the food, but vesical instillation yields the highest number of urinary bladder tumours, because this substance is directly decomposed and activated when exposed to acidic urine (Kunze & Gassner,1986; Kunze & Chowaniec,1990). N-butyl-N-(4-hydroxybutyl) nitrosamine is, no doubt, the most frequently used compound to experimentally induce urinary bladder tumours in small rodents, since it specifically targets this organ and is easy to use under experimental conditions (Druckrey,1964; Lijinski,1992). It is most commonly administered in the drinking water, although gavage administration may also be used (Oliveira et al. 2006). Intravesical instillation and subcutaneous administration of N-butyl-N-(4-hydroxybutyl) nitrosamine are also feasible alternatives (Cohen,1998; Oliveira et al. 2006) and yield identical incidences of urothelial lesions. Rats and mice exposed to N-butyl-N-(4-hydroxybutyl) nitrosamine develop a set of lesions very similar to those described in humans (Vasconcelos-Nóbrega et al. 2012). Ptaquiloside, a DNA-alkylating agent isolated from bracken (Pteridium aquilinum), is known for its ability to induce neoplastic and pre-neoplastic lesions of the urinary bladder in rats, mice and other domestic and laboratory animals. However, ptaquiloside also targets other organs, such as the ileum in the rat and the haematopoietic tissue in mice and is particularly difficult to isolate from bracken, which makes it unsuitable for the purposes of experimentally inducing urinary bladder tumours (Gil da Costa et al. 2011).
The rodent’s lower urinary system
The structure and functions of rodent’s lower urinary system are similar to those observed in humans (Arentsen et al. 2009). The lower urinary system includes the renal pelvis, ureters, urinary bladder and urethra (Squire,1998). The urinary bladder is a pear-shaped hollow organ, placed in the posterior abdominal cavity in the midline of the body, ventral to the descending colon (Komárek,2000; Krinke & Weber,2012). In rodents, the urinary bladder may be divided into dorsal and ventral areas, as well as the blind dome which is referred to as the fundus or vertex. The urinary bladder is attached to the ventral body wall by the ventral ligament (Frith et al. 1995). It temporarily retains the urine until it is excreted, and has the ability to adapt to considerable changes in volume (Kuehnel,2003). The thickness of the urinary bladder wall thus varies, depending on the degree of distension induced by urine accumulation.
Histologically, the lower urinary system is composed of three layers, known as mucosa, muscularis and serosa (Junqueira & Carneiro,2004). The mucosa is composed of a transitional epithelium, also known as urothelium, supported by a lamina propria composed of variably dense connective tissue. The urothelium is composed of three cell layers: basal, intermediate and superficial (Cohen,1998; Oyasu,1995), and is characterized by a low cellular proliferation index (Clayson & Cooper,1970; Kuehnel,2003). The superficial layer consists of large umbrella cells, which may be binucleated and have a polyploid DNA content (Krinke & Weber,2012). The lamina propria may show lymphoid aggregates, especially in older animals, which should not be mistaken for inflammatory lesions (Krinke & Weber,2012). The muscularis has an internal longitudinal and an external circular layers. The serosa shows a typical mesothelium supported by a slender connective tissue layer (Junqueira & Carneiro,2004).
Urine collection
Urine is a dynamic fluid and the main excretion route for endogenous and exogenous toxicants. Thus, urine reflects not only the function of the urinary system but also the ingestion of food and water, and metabolic alterations, changing its composition during the 24 hour cycle. During the night, rodents are more active and increase food and water intake, increasing the concentration of several urinary components. The concentration of these substances then decreases rapidly during the day, when animals are less active (Cohen et al. 2007). Bearing this in mind, it is important to collect urine samples from all animals consistently at the same time (Cohen,1995; Fisher et al. 1989). Collection of urine from experimental animals is an indispensable requirement in several urological studies. Different methods may be selected to collect urine samples according to the purpose of each experiment: spontaneous micturition, manual urinary bladder compression, cystocentesis, urinary bladder catheterization and the use of metabolic cages (Table1). Urine should always be collected in a perfectly clean container and analysed as quickly as possible. To perform these procedures the handler must always wear gloves and adequate clothing.
Spontaneous micturition is usually performed during the handling of laboratory animals. The animals should be placed on a plastic surface outside the cage and the voided urine may be aspirated into microcentrifuge tubes using an adjustable air displacement pipette. According to Kurien et al. (2004) urine must be collected as quickly as possible (within 12 seconds). Another option is to restrain the animal with one hand and collect the spontaneously eliminated urine into a tube with the other one.
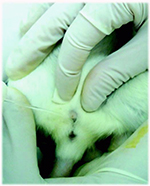
Manual urinary bladder compression induces forced micturition, the animal should be placed in dorsal recumbency. Then a firm but not traumatic compression is applied upon the urinary bladder in the supra-pubic region (Fig.1A). The sample may be collected into capillary tubes or other adequately sizedvials. This method allows the collection of small urine samples (150-200 µl from rat and 30-100 µl from mouse) (Weiss et al. 2000). If the aim is to obtain aseptic urine, the first urine drops should be rejected.
Cystocentesis is a very challenging technique in rats and mice. To perform cystocentesis, the animal should be sedated or anaesthetized and placed in dorsal recumbency, and the urinary bladder should be punctured through the abdominal wall. Routinely, cystocentesis must be performed only in the presence of a specific ultrasonography apparatus to avoid perforation of abdominal organs and sample contamination (e.g. with blood and intestinal content). In the absence of ultrasonography, this technique is only recommended immediately before the animal’s sacrifice and when sterile urine is absolutely necessary. This procedure carries significant risks of organ perforation and only yields small volume samples (Washington & Hoosier,2012; Weiss et al. 2000).
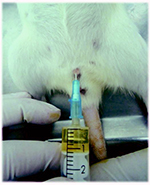
Another technique is urinary bladder catheterization. In order to perform this procedure (Fig.1B) the animal must be sedated/anaesthetized. This technique may only be performed in female laboratory rodents, since the male penile curvature hinders the introduction and progression of the catheter into the urinary bladder. The diameter of the catheter should be of 18 or 24 Gauge for rats and mice, respectively. Before introducing the catheter, the suprapubic region should be gently massaged in order to stimulate forced micturition. The first urine drops will lubricate the urethra and help to visualize the female external urethral meatus. The catheter should be sterile to minimize the risk of introduction of pathogens into the urinary system. Then, the flexible component of the catheter is inserted through the external urethral meatus (Oliveira et al. 2009). This technique carries risks for animals namely cystitis development and urethra or urinary bladder rupture (Sharp & La Regina,1998; Weiss et al. 2000).
Metabolic cages may be used when it is necessary to collect large volumes of urine (Fig.1C). The use of metabolic cages requires a period of adaptation by animals of between 24-48 hours, depending on the purpose of the study (Pires et al. 2010). The animal is individually confined and it is possible to collect urine and faeces separately, through a grid (Washington & Hoosier,2012; Weiss et al. 2000). The urine collected by this method may be contaminated by microorganisms.
Urine analysis
In the course of chemical carcinogenesis assays, urine may be collected for qualitative or quantitative analysis (Kurien et al. 2004). A small volume of urine (200 μl) is enough for qualitative urinalysis, which includes the urinary pH measurement, osmolarity, proteins, glucose, bilirubin, haemoglobin, ketone bodies, urobilinogen, creatinine and electrolytes (Suckow et al. 2001; Washington & Hoosier,2012). Quantitative excretion analysis for scientific purposes requires a 24-hour urine sample (Kurien et al. 2004). In order to analyse urine sediment, the sample (minimum volume 300 μl) should be centrifuged at 3000 rpm for 10 minutes, then the supernatant should be eliminated and the sediment, previously homogenized, placed on a microscope slide (Cohen et al. 2007). This sediment may contain crystals, cellular casts and isolated cells (Brimo et al. 2009). The identification of neoplastic cells in urine samples obtained during experimental urothelial carcinogenesis is only possible in advanced stages of neoplastic development.
Introduction
The use of laboratory animals contributes to biomedical research and to implement new preventive, diagnostic and therapeutic strategies. The selection of an adequate animal model requires careful and detailed planning, to ensure that experiments are technically feasible, reproducible and scientifically valid, and must also take into account ethical and legal issues concerning animal welfare.
The results obtained from animal experiments are in most cases extrapolated to humans. For this reason it is absolutely necessary to use correct techniques to collect and process the samples. Over the years, our research team has focused on urinary bladder cancer research, using rat and mouse models of chemical carcinogenesis. Analysing the available bibliography, we found that this subject is deficient in several areas, namely urine sample collection and recommendations for urinary bladder collection. Consequently, the aim of this work is to provide information about this subject and to give our experience acquired over the years.
In situ fixation and urinary bladder collection
The urinary bladder may only be collected at the end of an experimental protocol. To compare and extrapolate results between animal research protocols executed by different investigators, it is absolutely necessary to collect the urinary bladder under the same conditions. The urothelial carcinogenesis may be focal, multifocal or diffuse. Different stages of neoplastic development may be identified in the same urinary bladder. Consequently, it is necessary to examine multiple areas of the urothelial surface and the extent of the examinations should be equivalent among all animals and experimental groups (Squire,1998). If the urinary bladder is not distended, the urothelium will show artefactual folds that may be confused with papillary hyperplasia, a preneoplastic lesion. In situ urinary bladder distension is a meticulous procedure, and requires specialist knowledge, in order to avoid technical errors. If the purpose of the study is to evaluate the urinary bladder by scanning electron microscopy, the animal must be deeply anesthetized and the urinary bladder should be instilled with the fixative and collected while the animal is still alive. Autolytic changes are morphologically detectable by scanning electron microscopy within 60 seconds after the time of the animal’s death (Cohen et al. 2007).
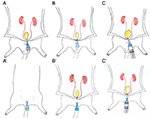
It should be noted that the urinary bladder collection procedure differs between male and female animals. In males, the procedure starts with an incision over the linea alba, from the xiphoid process to the pubis, taking care to not damage the underlying abdominal organs. An intravenous catheter is then introduced through the pelvic urethra (Fig.2A) and a loose ligature with a suture thread, preferably vinyl, is made around it, as closely as possible to the urinary bladder (Fig.2B). At this point, the needle is removed from the catheter and the fixative is inserted using a syringe (Fig.2C). The fixative volume depends on the animal species (100 and 300 µL for mice and rats, respectively). It is extremely important to distend the urinary bladder. The ligature is then tightened to avoid any reflux of the fixative and the malleable catheter is withdrawn. When working with female animals, the urinary bladder catheterization takes place before opening the abdomen, as described above in connection with urine collection (Fig.2A’). Only when the flexible component of the catheter is introduced into the urinary bladder is the abdominal cavity opened and the urinary bladder exposed. The following procedures to perform in situ female urinary bladder fixation and distension are similar to those described above for males (Fig.2B’, 2C’). After the introduction of fixative into the urinary bladder, the urethra should be cut, caudally to the ligature, and the urinary bladder immediately immersed into the fixative for 12 hours. Several fixatives may be used for urinary bladder preservation. For routine light microscopy, 10% neutral buffered formalin is recommended. However, the use of Bouin’s fixative is recommended by Cohen et al. (2007) for samples intended for scanning electron microscopy. If the purpose is to develop cell cultures, the urinary bladder must be collected under sterile conditions.
Macroscopic examination of the urinary bladder after fixation requires longitudinal bisection of the organ to obtain two equal halves and to inspect the urothelial surface. It is possible to identify diffuse or focal thickening or papillary growths. The most accurate approach is to map the lesions seen on macroscopic evaluation in each of the urinary bladder halves. Ideally this should be done with a magnifying glass and using light transmitted through the urinary bladder wall. Several methods have been described for the preparation of histological specimens. One that provides excellent assessment at multiple areas is a simple bisection followed by embedding both halves of the urinary bladder in the same paraffin block. Numerous slides may then be made, allowing examination of different sections of the bladder, each showing the entire circumference of the urinary bladder. Another method consists of multiple longitudinal strips of urinary bladder tissue embedded in paraffin in order to allow identification of all its layers on histological examination. However, this technique involves greater practice. Then, each section should be cut, in a microtome, to the appropriate thickness ( 2-5 μm). The most frequent staining used for microscopic evaluation is haematoxylin and eosin, however according to the research aims other stains may be used. Paraffin-embedded samples can also be investigated by using histochemistry, immunohistochemistry and molecular biology techniques (Oliveira et al. 2005; Vasconcelos-Nobrega et al. 2011).
Figure 1. Urine collection using different methods. A: Manual compression; B: Catheterization; C: Metabolic cage.
Click images to enlarge |
|
|
|
Figure 2. Schematic representation of urinary bladder collection in male (left) and female (right). A: Introduction of catheter in the pelvic urethra; B: Loose ligature with suture thread and needle removal; C: Fixative intravesical instillation. A’: Catheterization; B’: Loose ligature with suture thread; C’: Fixative intravesical instillation.
Click image to enlarge |
Table 1. Advantages and disadvantages of urine collection methods.
| Method | Advantages | Disadvantages |
| Spontaneous micturition | No need for sedation/anesthesia Easy to perform Non traumatic |
Small volume sample |
| Manual bladder compression | No need for sedation/anesthesia Easy to perform Aseptic samples Non traumatic |
Small volume sample |
| Cystocentesis | Aseptic samples | Risk of perforating internal organs Technically challenging Requires sedation/anaesthesia |
| Catheterization | Aseptic samples | Risk of introducing pathogenic agents into the bladder Only possible in females Requires sedation/anaesthesia |
| Metabolic cage | Collection of large urine samples over a defined period | Urine samples contaminated by microorganisms |
References
- Arentsen HC, K Hendricksen, E Oosterwijk & JA Witjes: Experimental rat bladder urothelial cell carcinoma models. World J. Urol. 2009, 27(3), 313-317.
- Becci PJ, HJ Thompson, JM Strum, CC Brown, MB Sporn & RC Moon: N-Butyl-N-(4-Hydroxybutyl)Nitrosamine-Induced Urinary-Bladder Cancer in C57Bl-6 X Dba-2F1 Mice As A Useful Model for Study of Chemoprevention of Cancer with Retinoids. Cancer Res. 1981, 41(3), 927-932.
- Brimo F, RT Vollmer, B Case, A Aprikian, W Kassouf & M Auger: Accuracy of Urine Cytology and the Significance of an Atypical Category. Am. J. Clin. Pathol. 2009, 132(5), 785-793.
- Clayson D & E Cooper: Cancer of the urinary tract. Adv. Cancer Res. 1970, 13271-381.
- Clayson DB, L Fishbein & SM Cohen: Effects of Stones and Other Physical Factors on the Induction of Rodent Bladder-Cancer. Food Chem. Toxicol. 1995, 33(9), 771-784.
Cohen SM: Role of Urinary Physiology and Chemistry in Bladder Carcinogenesis. Food Chem. Toxicol. 1995, 33(9), 715-730.
- Cohen SM: Monographs on pathology of laboratory animals , In: Urinary system. Introduction of cancer in the rat bladder: Pathogenesis, role of cell proliferation, and relevance to human disease . 2 Edit., Springer, USA. 1998, pp. 420-426.
- Cohen SM: Cell proliferation and carcinogenesis. Drug Metab. Rev. 1998, 30(2), 339-357.
- Cohen SM: Urinary bladder carcinogenesis. Toxicol. Pathol. 1998, 26(1), 121-127.
- Cohen SM: Comparative pathology of proliferative lesions of the urinary bladder. Toxicol. Pathol. 2002, 30(6), 663-671.
- Cohen SM, T Ohnishi, NM Clark, J He & LL Arnold: Investigations of rodent urinary bladder carcinogens: Collection, processing, and evaluation of urine and bladders. Toxicol. Pathol. 2007, 35(3), 337-347.
- Druckrey H: Selective induction of bladder cancer in rats by dibutyl- and N-butyl-N-butanol(4)-nitrosamine. Krebsforsch 1964, 2(66), 280-290.
- Fisher M, T Sakata, TS Tibbels, RA Smith, K Patil, M Khachab, SL Johansson & SM Cohen: Effect of sodium saccharinand calcium saccharin on urinary parameters in rats fed Prolab 3200 or AIN-76 diet. Food Chem. Toxicol. 1989, 27(1), 1-9.
- Frith C, JJ Eighmy, S Fukushima, SM Cohen, RA Squire & M Chandra: Proliferative lesions of the lower urinary tract in rats , In: Guide for Toxicologic Pathology. STP/ARP/AFIP , USA. 1995, pp. 1-13.
- Gil da Costa RM, PA Oliveira, M Vilanova, MM Bastos, CC Lopes & C Lopes: Ptaquiloside-induced, B-cell lymphoproliferative and early-stage urothelial lesions in mice. Toxicon 2011, 58(6-7), 543-549.
- Irving CC, WM Murphy & DS Daniel: Comparative Carcinogenicity of N-Butyl-N-(3-Carboxypropyl)-Nitrosamine and N-Butyl-N-(4-Hydroxybutyl)Nitrosamine for the Urinary-Bladder of (C57Bl/6Xdba/2)F1 Mice. J. Natl. Cancer I. 1984, 73(3), 753-756.
- Jemal A, F Bray, MM Center, J Ferlay, E Ward & D Forman: Global Cancer Statistics. Ca-A Cancer J. Clin. 2011, 61(2), 69-90.
- Junqueira L & J Carneiro: Aparelho urinário , In: Histologia básica. 10 Edit., Guanabara Koogan , Brasil. 2004, pp. 388.
- Komárek V: Gross anatomy , In: The laboratory rat . Academic Press, USA. 2000, pp. 253-277.
- Kompier LC, I Lurkin, MNM van der Aa, BWG van Rhijn, TH van der Kwast & EC Zwarthoff: Fgfr3, Hras, Kras, Nras and Pik3Ca Mutations in Bladder Cancer and Their Potential As Biomarkers for Surveillance and Therapy. Plos One 2010, 5(11),
- Krinke G & K Weber: Anatomy and normative biology , In: The laboratory mouse . 2 Edit., Elsevier, USA. 2012, pp. 190.
- Kuehnel W: Epithelial tissue , In: Color atlas of cytology, histology, and microscopic anatomy . 4 Edit., Thieme, USA. 2003, pp. 84.
- Kunze E & J Chowaniec: Pathology of tumours in laboratory animals. Tumours of the rat. Tumours of the urinary bladder . IARC Sci. Publ. 1990, 99345-397.
- Kunze E & G Gassner: Modification of N-Methyl-N-Nitrosourea-Induced Urinary-Bladder Carcinogenesis in Rats Following Stimulation of Urothelial Proliferation by A Partial Cystectomy. J. Cancer Res. Clin. 1986, 112(1), 11-18.
- Kurien BT, NE Everds & RH Scofield: Experimental animal urine collection: a review. Lab. Anim. 2004, 38(4), 333-361.
- Lijinski W: Chemistry and biology of N-nitroso compounds. Cambridge University Press, UK. 1992.
- Okajima E, T Hiramatsu, K Hirao, M Ijuin, Y Hirao, K Babaya, S Ikuma, S Ohara, T Shiomi, T Hijioka & H Ohishi: Urinary-Bladder Tumors Induced by N-Butyl-N-(4-Hydroxybutyl)Nitrosamine in Dogs. Cancer Res. 1981, 41(5), 1958-1966.
- Oliveira PA, A Colaco, PL De la Cruz & C Lopes: Experimental bladder carcinogenesis-rodent models. Exp.Oncol. 2006, 28(1), 2-11.
- Oliveira PA, C Palmeira, LM Lourenco & CA Lopes: Evaluation of DNA content in preneoplastic changes of mouse urinary bladder induced by N-butyl-N-(4-hydroxybutyl) nitrosamine. J. Exp. Clin. Cancer Res. 2005, 24(4), 609-616.
- Oliveira PA, MJ Pires, C Nobrega, R Arantes-Rodrigues, AM Calado, J Carrola, M Ginja & A Colaco: Technical Report: Technique of Bladder Catheterization in Female Mice and Rats for Intravesical Instillation in Models of Bladder Cancer. Scand. J. Lab. Anim. Sci. 2009, 36(1), 5-9.
- Oyasu R: Epithelial Tumors of the Lower Urinary-Tract in Humans and Rodents. Food Chem. Toxicol. 1995, 33(9), 747-755.
- Petraki CD & CP Sfikas: Non-papillary urothelial lesions of the urinary bladder: Morphological classification and immunohistochemical markers. In Vivo 2008, 22(4), 493-501.
- Pires MJ, A Rodríguez-Peña, A Colaço, M Arévalo, A Esteller & JM López-Novoa: Comparative effects of nebivolol and atenolol on renal function in rtas with chronic renal failure. Port. J. Nephrol. Hypert. 2010, 24(1), 33-43.
- Sharp P & M La Regina: Experimental methodology, In: The laboratory rat. CRC Press, USA. 1998, pp. 146.
- Squire R: Classification and differential diagnosis of neoplasms, urinary tract, rat , In: Monographs on pathology of laboratory animals, urinary system. Springer, USA. 1998, pp. 69-74.
- Suckow M, P Danneman & C Brayton: Important biological features, In: The laboratory mouse. CRP Press, USA. 2001, pp. 10-21.
- Vasconcelos-Nóbrega C, A Colaço, C Lopes & P Oliveira: Review: BBN as an urothelial carcinogen. In Vivo 2012, 26(4), 727-739.
- Vasconcelos-Nobrega C, A Colaco, L Santos, H Vala, LF Palomin, C Lopes & PA Oliveira: Experimental Study of the Anticancer Effect of Gemcitabine Combined with Sirolimus on Chemically Induced Urothelial Lesions. Anticancer Res. 2011, 31(5), 1637-1642.
- Washington I & G Hoosier: Clinical biochmestry and hemathology, In: The laboratory rabit, guinea pig, hamster, and other rodents. Elsevier, USA. 2012, pp. 61.
- Weiss J, G Tayler, F Zimmermann & K Nebendahl: Collection of body fluids, In: The laboratory rat. Academic Press, USA. 2000, pp. 494-498.