A case of bronchogenic adenocarcinoma in a guinea pig (Cavia porcellus)
by G. D. Leishangthem1, 2, 2 , P. D. Gadhave2, N. D. Singh2* & H. S. Banga2
1Department of Pathology, All India Institute of Medical Sciences
(AIIMS), New Delhi, India
2Department of Veterinary Pathology, College of Veterinary Science,
GADVASU, Ludhiana, Punjab, India.
 Correspondence: Dr. Nittin Dev Singh
Correspondence: Dr. Nittin Dev Singh
Department of Veterinary Pathology,
College of Veterinary Science, GADVASU, Ludhiana, Punjab, India.
Phone +91-161-2414027
Mobile +919780852132
e-mail drndsingh@gmail.com
Summary
A spontaneous case of bronchogenic adenocarcinoma in an adult female albino guinea pig characterized by presence of proliferative neoplastic bronchial epithelial cells forming nests, and vacuolation in the liver parenchyma, is reported.
Introduction
The guinea pig (Cavia porcellus) is an important laboratory animal for studies of infectious diseases, nutritional diseases and neoplasms; they are also popular pet animals (Flecknell, 2002). Spontaneous tumours are relatively uncommon in the guinea pig and they are mostly reported in older animals (Kitchen et al., 1975), especially in animals older than 3 years with an incidence rate as high as 30% (Greenacre, 2004). Tumours of the respiratory system and liver were previously reported by Rogers & Blumenthal (1960) and Vannevel & Wilcock (2005). Among the respiratory tumours, reports of naturally occurring bronchogenic adenocarcinoma in guinea pigs are very rare. In this communication, a case of bronchogenic adenocarcinoma in a female guinea pig which died naturally is reported.
Materials and methods
An adult female albino guinea pig which was kept at the Central Animal Facility, AIIMS, New Delhi, was found dead. A thorough necropsy examination was conducted, gross findings were recorded and organs showing lesions were collected in 10 % neutral buffered formalin and processed for routine histopathology and stained with hematoxylin and eosin (H&E) and Periodic acid Schiff (PAS) stain as per Bancroft & Gamble (2008).
Results
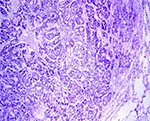
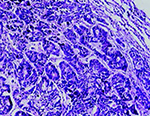
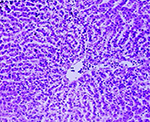
On post-mortem examination, numerous whitish nodules of variable size were diffusely distributed on the surface of the lungs exhibiting a disseminated pattern of spread of tumour. The liver was pale and enlarged and greasy on its cut surface. Histopathologically, the section of lung revealed, multifocal nodular growth with the involvement of bronchi and bronchioles. The tumour comprised large anaplastic cuboidal epithelial cells which had proliferated to form clusters or irregular acini or nests (Fig. 1). The tumour cells were hyperchromatic and arranged in single or multiple layers. Sloughed cells were found in the lumen of the tumour indicating the occurrence of metastasis. Adjacent alveolar spaces exhibited atelectasis and peripherally emphysema (Fig. 2). PAS staining showed no intracytoplasmic mucin secretion. The tumour showed the characteristic features of bronchogenic adenocarcinoma. In addition to the changes in lung, the liver showed moderate to severe microvesicular steatosis. The presence of vacuoles having peripheral nuclei, a characteristic of lipid accumulation was seen in the perilobular areas of the liver along with dilated sinusoids (Fig. 3). Various apoptotic hepatocytes were also seen in the liver parenchyma.
Discussion
To date only small numbers of spontaneous tumours have been observed
in guinea pigs which indicates that this species is probably one of
the mammals least prone to tumours.
Different types of spontaneous tumours including bronchogenic
papillary adenomas, carcinomas of the skin, trichofolliculomas, ovary
teratomas and tumours of the mammary glandhave been reported in guinea
pigs;the most common tumour of guinea pigs is bronchogenic papillary
adenoma
(Rogers & Blumenthal, 1960; Kitchen et al., 1975; Greenacre,
2004). Goldberg (1920) has reported a case of bronchogenic
adenocarcinoma in the guinea pig. Pulmonary adenomas and pulmonary
adenocarcinomas were found in guinea pigs with concomitant
interstitial pneumonia (Fischer, 1956). Our findings of
bronchogenic adenocarcinoma are in consonance with these reports.
The natural cancer resistance of the guinea pig has been shown by
studying spontaneous tumours as well as experimental cancer induction
with a number of carcinogenic agents
(Frank & Chasterman, 1962). Although it belongs to the
rodent class, the guinea pig reacts differently to that of other
rodents. Guinea pigs are considered to be a good model for better
understanding of the growth and development of respiratory system
tumours caused by carcinogenic substances or cigarette smoke
(Fiala et al., 2005). Fiala and his co-workers
(2005) induced preneoplastic lung lesions, including bronchial hyperplasia,
dysplasia and squamous metaplasia in guinea pigs.
Steatosis depends on an imbalance of fat intake and endogenous
lipogenesis, and also on the capability of the liver to oxidize fat
and deposit esterified fats and triglycerides intended for export in
the form of lipoprotein (Burt et al., 1988). In the guinea
pig, high concentrations of free cholesterol are present in the liver
(Angelin et al., 1992). They have moderate rates of hepatic
cholesterol synthesis (Reihner et al., 1990;
Fernandez et al., 1990) and catabolism (Reihner et al., 1991).
In our study, development of nodular growth in the lung parenchyma
causes anorexia and respiratory insufficiency. Anorexia may cause
mobilization of tissue fat reserve. Fatty changes in many animals have
been associated with periods of anorexia. Reduction in carbohydrate
intake and fat metabolism for energy leads to the development of fatty
changes in the liver of guinea pigs (Schaeffer & Donnelly, 1997). Restricted feeding triggers non-alcoholic fatty liver disease
(NAFLD) leading to perilobular steatosis of the liver
(Makovický et al., 2012). During starvation, liver cells
mobilize tissue fat reserves
(Hernandez-Espinosa et al., 2006) leading to fatty liver.
Moreover, fatty liver is also observed in guinea pigs fed on high
cholesterol and fat diet (Ye et al., 2013). It is presumed
that fat accumulation is due to inability of the parenchyma cells to
process excessive fat.
References
-
Angelin B, H Olivecrona, H Reihner, E Rudling, D Stahlberg, M
Eriksson, S Ewerth, P Henricksson. & K Einarsson:
Hepatic cholesterol metabolism in estrogen-treated men.
Gastroenterology, 1992, 103, 1657-1663.
-
Bancroft JD & M Gamble: Theory and Practice of
Histological Techniques. 6th edn. Churchill Livingstone, Elsevier,
China, 2008.
-
Burt AD, A Mutton & CP Day: Diagnosis and
interpretation of steatosis and steatohepatitis. Semin. Diagn.
Pathol., 1988, 15, 246-258
-
Fernandez ML, NY Yount & DJ McNamara: Whole body
cholesterol synthesis in the guinea pig: Effects of dietary fat
quality. Biochim. Biophys. Acta., 1990, 1044, 340-348.
-
Fiala ES, OS Sohn, C Wang, E Seibert, J Tsurutani, PA Dennis, K
El-Bayoumy, RS Sodum, D Desai, J Reinhardt & C Aliaga:
Induction of preneoplastic lung lesions in guinea pigs by cigarette
smoke inhalation and their exacerbation by high dietary levels of
vitamins C and E. Carcinog., 2005, 26 (3), 605- 612.
-
Fischer W: Adenomatosis and cancerous formations during
chronic penmonia of the guinea pig. Zentralbl. Pathol., 1956,
94, 555-562.
-
Flecknell P: Guinea pigs. In: Manual of Exotic Pets, ed.
Meredith A, Redrobe S, 4th ed., British Small Animal Veterinary
Association, Gloucester, UK., 2002, pp. 70–88.
-
Franks LM & FC Chasterman: The pathology of tumours and
other lesions of the guinea pig lung. Brit. J. Can., 1962,
14(4), 696-700
-
Goldberg SA: The occurrence of epithelial tumors in the
domesticated animals. J. Am. Vet. Med. Assoc., 1920, 58,
47-63.
-
Greenacre CB: Spontaneous tumors of small mammals. Vet.
Clin. North Am. Exot. Anim. Pract., 2004, 7, 627–651.
-
Hernández-Espinosa D, I Ayala, MT Castells, B García-Pérez, A
Martín-Castillo, A Miñano, I Arcas , V Vicente & J Corral: Intracellular retention of hepatic serpins caused by severe
hyperlipidemia. Liver Int., 2006, 26, 708-715.
-
Kitchen DN, WW Carlton & AA Bickford: A report of
fourteen spontaneous tumors of the guinea pig. Lab. Anim. Sci.,
1975, 25, 92–102.
-
Makovický P, E Tůmová, R Rajmon, Z Bízková & H Härtlová:
The influence of restrictive feeding of chickens on the microscopic
structure of their liver. Acta Vet. Brno., 2012, 81,
027–030.
-
Reihner E, B Angelin, I Bjorkhem & K Einarsson:
Hepatic cholesterol metabolism in cholesterol gallstone disease. J.
Lipid Res., 1991, 32, 469-475.
-
Reihner E, B Angelin, M Rudling, S Ewerth, I Bjorkhem & K
Einarsson:
Regulation of hepatic cholesterol metabolism in humans: Stimulatory
effects of cholestyramine on HMG-CoA reductase activity and low
density lipoprotein receptor expression in gallstone patients. J.
Lipid Res. 1990, 31, 2219-2226.
-
Schaeffer DO & TM Donnelly: Disease
problems of guinea pigs and chinchillas, p.
260-281. In E. V. Hillyer and K. E. Quesenberry
(ed.), Ferrets, rabbits, and rodents: clinical medicine and
surgery. W. B. Saunders Co., Philadelphia, Pa. 1997.
-
Rogers JB & Blumenthal HT: Studies of guinea pig
tumors: I. Report of fourteen spontaneous guinea pig tumors, with a
review of the literature. Cancer Res. 1960, 20, 191–197.
-
Russell-Lodrigue KE, GQ Zhang, DN McMurray & JE Samuel:
Clinical and Pathologic Changes in a Guinea Pig Aerosol Challenge
Model of Acute Q Fever. Infect. Immun., 2006,
74(11), 6085–6091.
-
Vannevel J & B Wilcock : Bile duct carcinoma and nasal
adenocarcinoma in a guinea pig. Can. Vet. J., 2005, 46,
72–73.
- Ye P, IK Cheah & B Halliwell: A high-fat and cholesterol diet causes fatty liver in guinea pigs. The role of iron and oxidative damage. Free Radic. Res., 2013, 47(8), 602-613.