NSG mouse strain, health monitoring and microbiological findings
by J Sparrowe1, L Jiménez1, A Talavante1, I Angulo2, A Martínez1
11Laboratory Animal Science,
2Malaria Therapeutic Efficacy
Diseases of the Developing World-Drug Discovery Centre. GSK
R&D. Severo Ochoa, 2. 28760 Tres Cantos, Madrid, Spain.
 Correspondence: John Sparrowe, GSK R&D S.L.,
Correspondence: John Sparrowe, GSK R&D S.L.,
Diseases of the Developing World-Drug Discovery Centre
P.T.M Severo Ochoa, 2 - 28760. Tres Cantos, Madrid, Spain
Mobile +34 609 570 874
Tel +34 91 80 70653
Email john.g.sparrowe@gsk.com
Summary
The NSG strain is one of the most immunodeficient mice available, and
provides an effective model for studies where the engraftment of human
cells is required. However because of their severe adaptive and
innate immune deficiency, these mice must be kept under high
environmental control standards.
Routine health monitoring, according to FELASA guidelines, included
antigen testing in NSG animals and antibody testing in sentinel
animals (negative results, not shown).
We found saprophytic flora, by use of classic microbiology techniques,
in a variety of tissues and organs. Many of the sampled animals were
found to have bacteria growing in the spinal cord, tarsal joint,
heart, spleen, liver, kidney or blood. In order to rule out possible
tissue contamination, we sent samples to three different external
laboratories. All the samples submitted came back with the same
results.
We postulate that the widespread presence of these saprophytic
bacteria may be due to a lack of IgA secretion at the mucosal
epithelium, and the bacterial growth in these tissues and organs to
the immunodeficiency including impaired macrophage activity. The
potential clinical significance of these bacteria in NSG mice, if any,
is not known. To explore the possible connection between the bacterial
infection and animals with signs of slight limb paresthesia (numbness)
and paralysis, and arthritis, seen in 0.5-1% of animals, further
studies are needed.
Introduction
NSG mice are severely immunocompromised animals. Their main features
are absence of mature T or B cells, lack of functional NK cells and
deficiency in cytokine signaling. Immunological cells present in these
immunodeficient mice are neutrophils, monocytes, macrophages and
dendritic cells, though the last two are hypofunctional (Schultz
et al., 2005).
The NSG mouse strain provides an invaluable tool to accelerate the
development of potential therapies for the treatment of malaria in a
Humanized Mouse Model of Plasmodium falciparum malaria at our
Institution (Jiménez-Díaz et al., 2009).
Despite the high immunodeficiency of these mice, when maintained
appropriately they do not show health problems. The only clinical
sign that we have observed has been a mild paresthesia with paresis of
the digits in both the fore and hind limbs, and associated arthritis,
in 0.5-1% of the animals. These clinical signs were detected upon the
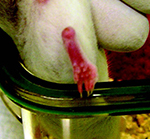
animals’ arrival at our laboratory (Figure 1).
|
Figure 1. Right tarsal joint arthritis
Click image to enlarge |
Materials and methods
Animals
NSG (NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ) mice were purchased
from Jackson Laboratories and Charles River Laboratories, and were
maintained under maximum barrier conditions.
All animal studies were ethically reviewed and carried out in
accordance with European Directive 2010/63/EU and the GSK Policy on
the Care, Welfare and Treatment of Animals.
Health Monitoring
Routine health monitoring of the NSG strain was performed on 43 naive
NSG mice. These were randomly selected on arrival, with uncrating of
the boxes within a Class II biological safety cabinet, in a laboratory
external to our facility. After housing the animals inside our
facility, seven mice showing swollen tarsal joints and/or gait
alterations were immediately isolated and also underwent the same
health monitoring procedure within the following 24 hours. The program
included routine extended FELASA profile, with necropsies, and
detection of pathogens’ antigens including pathogenic bacteria
by PCR, RT-PCR and culture.
NSG strain health was also screened by classic microbiological culture
of samples, aseptically taken, and individually processed and
cultured, from: oral cavity and cecum by swabbing, spinal cord by
blowing the vertebral canal using sterile syringes, and from lung,
heart, liver, kidney, spleen, tarsal joint and blood. Samples were
checked for the following opportunistic and commensal agents:
Streptococcus α –haemolyticus, Enterococcus, coagulase
positive and negative Staphylococcus, Lactobacillus spp,
Corynebacterium spp, Coliform LFC’s (E.coli,
Klebsiella spp, Enterobacter spp,
Citrobacter spp…), Coliform NLFC’s (Proteus spp, Serratia...), and water transmitted microorganisms (Pseudomonas spp,
Alcaligenes spp, Acinetobacter spp).
Necropsies were performed at the Madrid School of Veterinary Medicine,
Universidad Complutense. Swabs and samples were kept in AMIES
transport medium at 4 °C and were sent refrigerated at 4-8 °C to three independent international diagnostic laboratories that
used API VITECK 2 - a system designed primarily to identify human and
animal bacterial pathogens - for bacterial growth, isolation of
primary cultures and identification.
Results
Healthy animals (control group)
All animals were negative for the FELASA list of pathogens. Regarding
the microbiological culture work looking for saprophytic or
opportunistic agents, the results of the three external laboratories
were in concordance. Growth of many commensal bacterial strains was
found in almost all tissues sampled, in a high percentage of the
animals, without a predominant bacterial strain pattern. The most
frequent location with growth was the spinal cord.
List of microorganisms isolated in each tissue in order of frequency:
Spinal cord: E. coli (7 mice), Strep. mitis (5
mice), Staph. coag. neg. (5 mice),
Lactobacillus spp (4 mice), A. viridans (3 mice),
Staph. aureus (2 mice), E. faecalis (2 mice),
E. cloacae (1 mouse) and E. faecium (1 mouse);
lungs: E. coli (1 mouse) and Lactobacillus spp (1
mouse); heart: E. faecalis (4 mice),
Lactobacillus spp (2 mice), Staph. aureus (1 mouse),
E. coli (1 mouse) and Strep. mitis (1 mouse); liver:
Strep mitis (3 mice), Lactobacillus spp (3 mice),
Staph. aureus (1 mouse), A. viridans (1 mouse) and
E. faecalis (1 mouse); kidney: Lactobacillus spp (5
mice), E. faecalis (3 mice), Staph. coag. neg. (3
mice), A. viridans (2 mice) and E. coli (2 mice);
spleen: E. coli (3 mice), Lactobacillus spp (2
mice), Staph. aureus (1 mouse), Strep. mitis (1
mouse), E. faecium (1 mouse) and E. faecalis (1
mouse); tarsal joint: E. coli (2 mice),
Strep. mitis (1 mouse), E. faecium (1 mouse) and
Lactobacillus spp (1 mouse); blood: Staph. aureus (1
mouse), Strep. mitis (1 mouse), E. faecalis (1
mouse) and Staph. coag. neg. (1 mouse).
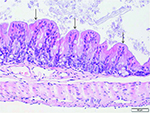
The histopathology report also stated that there were multifocal and
marked bacteria adherent to the mucosal surface in cecum and colon of
all the animals (Figure 2).
|
Figure 2. Bacteria adherent to the mucosal surface of colon, multifocal, marked.
Click image to enlarge |
Animals with clinical signs
The same procedure was performed on seven animals showing slight limb
numbness and paralysis and arthritis. The outcome was different, as
bacteria were found less frequently, particularly in the spinal cord.
List of microorganisms isolated in each tissue in order of frequency:
Spinal cord: E. coli (1 mouse), Strep. mitis (1
mouse) and G. morbillorum (1 mouse); these three findings
refer to the same individual; liver: G. morbillorum (1
mouse); spleen: G. morbillorum (1 mouse); all the other
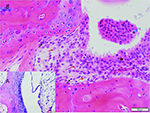
tissues were culture negative. The pathologist also found arthritis in
the tarsal joint or thickening of joint capsule (Figure 3), and the
same observations in cecum and colon mucosa.
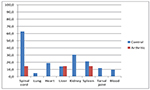
The percentage of mice with bacterial growth in various organs was
higher in NSG mice without clinical signs (control group), see Figure
4. The percentage of mice with bacteria in the spinal cord was
particularly high in the control group.
Discussion
All the bacterial strains found in tissues were also isolated and
identified in cecum and/or oral cavity. These bacterial strains can be
considered commensal or opportunists. One of the opportunists found
was Staph. aureus. It was isolated from spinal cord, heart,
liver and spleen, identified by molecular detection, and sequenced for
confirmation in three control animals of the same batch, but only in
one out of a large number of shipments. Although pathogenicity could
be expected due to the many potential virulence factors of
Staph. aureus, no differences were shown in the health of NSG
mice from the same group. . The list of organisms monitored at the
supplier’s breeding colony, as shown in their animal health
reports under the “other organisms monitored” section,
includes Staphylococcus aureus. This “other
agents” monitoring agrees with the FELASA recommendations for
the health monitoring of immunodeficient animals (Mähler
et al., 2014); it indicates the necessity to monitor such
animals for opportunists or commensals, but also acknowledges that to
define a complete list is impossible.
Although the reason for the bacterial colonization of the tissues of
NSG mice is understandable, it is unclear whether it should be
considered an infection. The absence of functional B cells implies a
zero level of immunoglobulin. Thus mucosal barriers do not have IgA
dimers (Foreman et al., 2011) in mucosal secretions to
control the spread of bacteria across this barrier.
These mice also lack NK cells and T lymphocytes, so survival of
invading microorganisms is favoured. The presence of bacteria in
normally sterile body sites did not correlate with inflammation.
Despite the serious immunological impairment, these mice did not show
morbidity, other than a few cases of mild arthritis. Their good health
can be explained by the non pathogenic nature of the
saprophytic/opportunistic bacteria and the absence of a host reaction.
There seems to be some natural tropism of bacteria for spinal cord
tissue in the control group, with a much higher incidence of bacteria
in the spinal cord than in the mice showing signs of
arthritis/paralysis. It can be hypothesized that, although
immunologically impaired, it is likely that phagocytic activity was
involved in eliminating bacteria from the mice with clinical signs (Hu
et al., 2011). It is possible that increased levels of Il-6
and other proinflammatory cytokines, secreted by phagocytes and by
dendritic cells involved in killing the bacteria might explain the
clinical signs seen in these animals (Liang et al., 2009).
Conclusions
The addition of microbiological culture of tissues to the health
monitoring routine for immunodeficient strains showed that the normal
condition of the NSG strain, when they arrive at our facility, is to
contain some amount of normal flora and various opportunistic
bacterial strains disseminated throughout their bodies, despite a low
incidence of clinical signs or lesions found in tissues.
To explore the possible connection between these bacteria, an altered
immune response to them and animals with signs of slight limb numbness
and paralysis, and arthritis, further studies are needed.
Acknowledgements
The authors wish to thank our colleagues Violeta Solis, Greg Whelan and Jeff Burdick, for their review, advice and comments.
References
-
Foreman O, AM Kavirayani, SM Griffey, R Reader & LD
Shultz: Opportunistic bacterial infections in breeding colonies of the
NSG mouse strain. Vet. Pathol.,2011, 48, 495–499.
-
Hu Z, N Van Rooijen & YG Yang: Macrophages prevent
human red blood cell reconstitution in immunodeficient mice. Blood,
2011, 118(22), 5938-5946.
-
Jiménez-Díaz MB, T Mulet, S VieraS, V GómezV, H Garuti, J
Ibáñez, A Alvarez-Doval, LD Shultz, A Martínez, D Gargallo-Viola
& I Angulo-Barturen: Improved murine model of malaria using P. falciparum competent
strains and non-myelodepleted NOD-scid IL2R {gamma}null mice
engrafted with human erythrocytes. Antimicrob. Agents Ch., 2009,
53(10), 4533-6.
-
Liang B, Z Song, B Wu, D Gardner, D Shealy, XY Song & PH
Wooley: Evaluation of anti-IL-6 monoclonal antibody therapy using murine
type II collagen-induced arthritis. J. Inflamm., 2009,
6, 10.
-
Mähler M, M Berard, R Feinstein, A Gallagher, B Illgen-Wilcke, K
Pritchett-Corning & M Raspa: FELASA recommendations for the health monitoring of mouse, rat,
hamster, guinea pig and rabbit colonies in breeding and experimental
units. Lab. Anim., 2014, 48(3), 178–192.
- Shultz LD, BL Lyons & LM Burzenski: Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2rgnull mice engrafted with mobilized human hematopoietic stem cells. J. Immunol. 2005, 174,6477-6489.