Original scientific article
A modified technique for thymectomy in adult mouse with no ventilation support
by Chenchen Zhao1, Hui Zeng2, Zhengya Yu3
1Department of General Surgery, Beijing Tongren Hospital, Capital
Medical University, Beijing 100730, China
2Institute of Infectious Diseases, Beijing Ditan Hospital, Capital
Medical University, Beijing 100015, China
3Department of General Surgery, Beijing Tongren Hospital, Capital
Medical University, Beijing 100730, China
 Correspondence: Zhengya Yu, Department of General Surgery
Correspondence: Zhengya Yu, Department of General Surgery
Beijing Tongren Hospital, Capital Medical University
No.1 Dong Jiao Min Xiang Street, Dongcheng District, Beijing 100730,
China
Tel/Fax +86-10-58268504
Email zhengya_yu@ccmu.edu.cn
Summary
Thymectomy in adult mice, a method to eliminate newly produced naïve T
cells, has proved valuable in various immunological studies. However,
pneumothorax is the primary cause of high mortality while preforming
the traditional thymectomy technique. Although modified techniques
utilizing mechanical ventilation support can reduce the occurrence of
pneumothorax, it may increase operational complexity and cause
ventilation-induced lung injury, which could interfere with subsequent
investigations by causing inflammation and a series of immune
responses. To solve this problem, we developed a novel technique using
pleural ligations to replace ventilation support before thymus
removal. Our method not only reduced the incidence of pneumothorax but
also caused less disturbance to the immune system. No thymic residue
was found by postoperative autopsy and a distinct decrease of T cells
in peripheral blood was detected by flow cytometry analysis. The
mortality rate was 3.3%, which is comparable to the
ventilation-support technique, yet the new procedure is simpler and
faster. This technique provides both reliability and simplicity for
thymectomy in adult mice.
Introduction
Thymectomy has been widely used as an effective method for studying the function of the thymus and T cells in various fields, such as infection, transplantation and tumor immunity etc. As distinct from thymectomy in neonatal mice and genetically engineered mice such as SCID and nude mice, in which T cells are depleted at a very young age, thymectomy in adult mice can create a unique immune status at a selected time at which no newborn T cells are released to the circulation (Miller, 1965). In mice, naïve T cells are almost exclusively sustained by thymus output ( den Braber et al., 2012 ). Thus, this method may not only help researchers to manipulate the T cell pool according to their interests, but also be an effective tool to study the role of the thymus under different pathological conditions.
The original technique of thymectomy in adult mice was described in 1963, which mainly used suction to remove thymus lobes (Sjodin et al., 1963). However, there were two shortcomings that rendered this method unsatisfactory. Firstly, suction may not assure complete removal of the thymus, because suction through the constrictive tube could tear the thymus into pieces. Secondly, the mortality rate was high, up to 5-30% (Castro JE, 1974; DeMatteo et al., 1995; Vrisekoop et al., 2008). Pneumothorax was the primary cause of death followed by hemorrhage of the heart or mediastinal vessels (DeMatteo et al., 1995). Our experience is that about 20-30% of mice succumb to this procedure (unpublished data). To overcome this, researchers developed a method using intubation and mechanical ventilation to prevent respiratory failure (DeMatteo et al., 1995). Despite the mortality rate decreasing to 3-6%, this modified method not only prolonged the operating time, increased complexity and added experimental cost, but also resulted in unexpected negative consequences such as ventilation lung injury. The aim of the present study was to develop a new method, which provides complete removal of thymus tissue, as well as improving animal survival with minimal side effects.
Materials and methods
Mice. Thirty C57BL/6J male mice
(6-8 weeks old, weighing 19-21g) were purchased from the
Institute of Experimental Animals, Chinese Academy of Medical
Science (Beijing, China). To avoid fighting, mice from the same litter
were housed in groups of five in polypropylene cages
(290×178×160mm) with sufficient specific pathogen free (SPF) soft
wood shavings (GB14924.3-2010, HFK bioscience, Beijing, China). Cotton
wool was added to the shavings to help the mice nesting, and
disposable cardboard boxes were provided for hiding and occupation,
which might also prevent aggressive behavior. Mice were given
ad libitum access to double-distilled water and commercial
SPF pelleted food (GB14924.3-2010, HFK bioscience, Beijing, China).
All animals were kept at a room temperature of 24±2 o C and
50±10% relative humidity on a 12h light/dark photoperiod. All
procedures performed on animals were approved by the Animal Care
Research Ethics Committee of the Capital Medical University of
China.
Equipment. Stereomicroscope (
Zeiss Stemi 2000-C, Oberkochen, Germany ), 6-0 silk braided
non-absorbable suture (Ethicon, Johnson-Johnson, NJ, USA), and 7-0
polypropylene suture (Ethicon, Johnson-Johnson, NJ, USA).
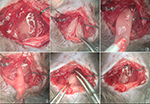
Procedure of thymectomy in adult mouse. All operations were
performed in a special disinfected operating room. Surgical
instruments were sterilized and disposable sterile gloves were used
during the operation. Mice were anesthetized by intraperitoneal
injection of narcotic fluid (ketamine 100mg/kg, 10mg/kg xylazine
0.1ml/10g), and placed on a dissecting board in supine position. The
feet of the mice were fixed with tape to keep a stable position
during the operation. The skin of neck and upper chest was
clipped and disinfected with
70% alcohol. Under a stereomicroscope, a 15-20mm skin incision
was made along the anterior median line from the suprasternal notch
to the level of the third ri b. Then, the suprasternal fossa was
exposed by dividing the superficial fascia and moving the submaxillary
glands aside. A central sternal incision was made to the level of
the second rib using microdissection scissors. In order to
prevent pneumothorax and hemorrhage, this incision was made
strictly along the midline with the scissor tips tilting
towards the dorsal aspect of the sternum (Figure1, step
1). The bisected sternum was retracted laterally with 6-0 silk
braided sutures implanted on the bilateral edges in order to expose
the mediastinum. Suturing was performed from interior to exterior
to avoid punctures inside the chest cavity. Such punctures might
easily have led to pneumothorax, even hemorrhage and
organ damage (Figure 1, step 2). Two white thymus lobes
were exposed. Each lobe was enveloped separately in a sack-like thin
membrane connected to the pleura, which was hard to discern. Careful
dissection was performed to open the sack and separate the lobe
from its membrane without damaging the pleura. The right lobe
was gently held and drawn by iris forceps and the inferior
pole of the lobe was exposed (Figure1, step 3). To perform
pleura ligation at the bottom of the thymus lobe, a 7-0 polypropylene
suture loose knot was made in advance to avoid making risky
movements inside the chest cavitywhile tying knots. Then the
circle was set at the caudal edge of the lobe (Figure1, step 4). After
the pleura and the nourishing blood vessels of the lobe were
ligated, the entire lobe was cut along the edge and removed carefully
under clear vision (Figure1, step 5). The left lobe of the
thymus was removed in a similar manner. The chest cavity was
checked for thymic remnants and accessory injuries before
being sealed with 6-0 silk suture lines, which were formerly used
for sternal retraction (Figure1, step 6). After the skin had been
approximated with single silk sutures or wound clips, the
incision was cleaned with 0.9% physiological saline. Following
thymectomy, animals were given fentanyl citrate (2.5 μg /kg,
sc.) and placed in clean cages in groups of five with warming lights
until they recovered from anesthesia. Mice were kept for 14 days until
euthanized for examination.
Post-operation assessment. To test the success of the thymectomy, autopsy and flow cytometry analysis were performed 14 days after the operation. With the help of narcotic fluid, mice were deeply anesthetized and blood samples were collected from the orbital sinus into heparinized tubes. Then, mice were euthanized by cervical dislocation. After the diaphragm had been dissected, the thoracic cavity was exposed under a stereomicroscope in order to search for thymic residues. In case of small thymic residues invisible to the naked eye, we harvested all soft tissue from the original position of the thymus and stained the cells with fluorescein isothiocyanate (FITC)-conjugated anti-mouse CD45, PE-conjugated CD8 and APC-conjugated CD4 antibodies (BD Bioscience, San Diego, CA , USA). We next detected the influence of thymectomy on peripheral blood. After erythrocyte lysis with BD Pharm Lyse solution (BD Bioscience, Sparks, MD, USA), blood cells were stained with phycoerythrin (PE)-conjugated anti-mouse CD8 and allophycocyanin (APC)-conjugated anti-mouse CD4 antibodies (BD Bioscience, San Diego, CA , USA). All cells were examined tested by a BD FACSCalibur flow cytometer (BD, CA , USA). Flow cytometry data were analyzed using Flowjo (7.6.5, Tree- Star Inc., OR, USA) and Prism (6.0c, GraphPad Software Inc., CA , USA). Data were analyzed by using the Student t test. P<0.05 was defined as significant.
Results
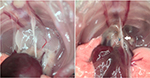
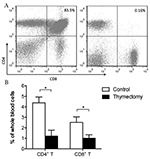
By using legation thymectomy, the mortality rate was 3.3% (1 of 30). Only one animal succumbed to severe pneumothorax during sternotomy. All other mice had successful operations and lived until the endpoint of the experiment with no postoperative complications (such as tachypnea, wound infection etc.). Autopsy confirmed that no residue or regenerated tissue of thymus was observable in the mediastinum (Figure 2, blue polypropylene knots were still visible). No signs of pulmonary or cardiovascular injury were observed. Results from flow cytometry showed that no group of CD4/CD8 double positive cells was found in tissue harvested from the former thymus location (Figure 3A), and a significant decrease of CD4 + T and CD8 + T cells in peripheral blood was found (P <0.01) (Figure 3B). Results from both autopsy and flow cytometry indicated a complete removal of thymus. Moreover, the average time of the operation for each mouse was around ten minutes in our laboratory.
Discussion
The primary challenge of thymectomy in adult mice is
manipulation of the delicate pleura. Animals can suffer from
instant death due to anoxia and mediastinal flutter triggered
by the change in intrapleural pressure. In such cases, researchers use
mechanical ventilation to support pulmonary function and prevent
progression of small pneumothorax (
DeMatteo et al., 1995 ). Although this method seems to be a
successful solution, the extra apparatus and procedural steps
needed may render the whole method too complicated. Moreover,
ventilation support could lead to ventilation-induced lung injury
(VILI) including collapse of alveolar units, edema and
inflammation etc. In particular, VILI has been associated with
increased bronchoalveolar lavage fluid (BAL) levels of cytokines such
as TNF-α and IL-6, and chemokines such as macrophage
inflammatory protein (MIP)-2 (Chiumello et al., 1999; Cheng et al., 2002). Both impaired pulmonary function and inflammation might adversely
affect subsequent immunological experiments. To eliminate these
problems, it is necessary to develop an innocuous technique
free from ventilation support and with high reliability as well.
We noted that the inferior pole of the thymus lobe was intimately
attached to the pleura and cardiac pericardium with several nourishing
blood vessels embedded. Because the pleura was more soft and fragile
than blood vessels, it was easier to tear while blood vessels were
still attached to thymus lobes. A small pleural leakage could quickly
be enlarged by chest movement and become fatal. We also observed that
in most failed cases, pneumothorax often occurred when the inferior
pole of the thymus was pulled or bluntly dissected. In fact, strong or
sudden traction of thymus lobes could even lead to aorta and atrium
dislocation and rupture. Hence, thymectomy with strong direct
traction, such as suction described in the conventional
technique, may not be appropriate. Furthermore, the sucking force
is difficult to control and the cannula tip needs fine
adjustment. Suitable modification of the cannula tip is extremely
difficult because the pleura is likely to be involved during suction
using a large diameter cannula, yet thymic residue is hard to avoid
since a small diameter tip could easily tear tissue into pieces
instead of removing the complete thymus.
To maintain the integrity of the pleura, we abandoned suction
thymectomy and introduced ligation thymectomy by ligating the pleura
at the bottom of thymus lobes. Firstly, ligation could prevent small
pleural leakage by tying up the nourishing blood vessels with
surrounding pleura, which could greatly minimize the risk of
pneumothorax. Secondly, thymus excision is more reliable and safer
with ligation, because the edge of the thymus lobe is clearly visible.
In this way, complete removal of the thymus is ensured.
Post-operation examination also confirmed that no residue of thymus
remained. For better operational sight and handling, we
strongly suggest this procedure be performed with
the help of a stereomicroscope and microinstruments.
This method of thymectomy in adult mice is as efficient as
procedures with ventilation support yet it is simpler, easier and
cheaper. More importantly, there are no ventilation-induced injuries
to the respiratory system and subsequent immunological disturbance is
eliminated. Our method conforms to the concept of the 3Rs
(replacement, reduction, refinement) and provides a stable animal
model for future immunological investigation.
Acknowledgements
This work was supported by the Innovative Research Program of Capital
Medical University, Beijing, China (No. xsky2012089).
Authors declare no competing interests.
References
-
Castro JE: Surgical procedures in small laboratory animals. J. Immunol. Methods., 1974, 4, 213-216.
-
Cheng KC, H Zhang, CY Lin & AS Slutsky: Ventilation with negative airway pressure induces a cytokine response in isolated mouse lung. Anesth. Analg., 2002, 94, 1577-1582, table of contents.
-
Chiumello D, G Pristine & AS Slutsky: Mechanical ventilation affects local and systemic cytokines in an animal model of acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med., 1999, 160, 109-116.
-
DeMatteo RP, JF Markmann & SE Raper: An improved technique of thymectomy in the adult mouse. Transplantation, 1995, 59, 787-789.
-
den Braber I, T Mugwagwa, N Vrisekoop, L Westera, R Mögling, AB de Boer, N Willems, EHR. Schrijver, G Spierenburg & K Gaiser: Maintenance of peripheral naive T cells is sustained by thymus output in mice but not humans. Immunity, 2012, 36, 288-297.
-
Miller JF: Effect of thymectomy in adult mice on immunological responsiveness. Nature, 1965, 208, 1337-1338.
Sjodin K, AP Dalmasso, JM Smith & C Martinez: Thymectomy in Newborn and Adult Mice. Transplantation, 1963, 1, 521-525.
-
Vrisekoop N, I den Braber, AB de Boer, AF Ruiter, MT Ackermans, SN van der Crabben, EH Schrijver, G Spierenburg, HP Sauerwein, MD Hazenberg, RJ de Boer, F Miedema, JA Borghans & K Tesselaar: Sparse production but preferential incorporation of recently produced naive T cells in the human peripheral pool. Proc. Natl. Acad. Sci. USA, 2008, 105, 6115- 6120.