Original scientific article
Lactobacillus acidophilus: effects on the pharmacokinetics of marbofloxacin in rats
by BT Birhanu1, N-H Park1, J-Y Park1, S-J Lee1, S-P Lee2, J-W Suh3,*, S-C Park1,*
1Laboratory of Veterinary Pharmacokinetics and Pharmacodynamics,
College of Veterinary Medicine, Kyungpook National University,
Bukgu, Daegu, 41566, South Korea
2The Center for Traditional Microorganism Resources (TMR), Keimyung
University, Daegu 704-701, South Korea
3Center for Nutraceutical and Pharmaceutical Materials, Division of
Bioscience and Bioinformatics, Science campus, Myongji University,
San 38-2, Namdong, Cheoin-Gu, Yongin, Gyeonggi 449-728, South
Korea
 Correspondence: Seung-Chun Park (parksch@knu.ac.kr).
Correspondence: Seung-Chun Park (parksch@knu.ac.kr).
Tel: 82-53-950-5964. Fax: 82-53-950-5955.
Joo-Won Suh: jwsuh@mju.ac.kr,
Tel 82-31-330-6881, Fax: 82-31-321-7361
Summary
Background: Probiotics are currently produced
commercially and widely used for improving human and animal health.
They modulate the gut environment through secretion and production of
different molecules and enzymes. Hence, they play a major role in
changing the pharmacokinetics of an orally administered drug.
Purpose: To determine the effect of
Lactobacillus acidophilus (KCTC 3140) on the pharmacokinetics
of marbofloxacin.
Materials and Methods: Five male and five female
8-week old healthy Sprague Dawley rats were treated with 109 CFU/ml of
L. acidophilus daily for seven days via the intra-gastric
route. Marbofloxacin (20 mg/kg) was administered orally at the
beginning and the end of the experiment. The plasma concentration of
marbofloxacin was measured using high performance liquid
chromatography (HPLC).
Results: The area under the curve (AUC) of
marbofloxacin declined from 5.78 µg.h/ml to 2.57 µg.h/ml after
treatment with L. acidophilus. Similarly, the maximum plasma
concentration (Cmax) of the drug decreased from 2.4 µg/ml
to 1.2 µg/ml and the Tmax increased from 0.54 to 0.73 h. The
elimination half-lives of marbofloxacin before and after treatment
with L. acidophilus were 1.19 h and 0.69 h, respectively. The
study was conducted by separating the male and female rats; no
significant difference was observed between the sexes.
Conclusion: The treatment of rats with
L. acidophilus decreased the plasma AUC and Cmax
after oral administration of marbofloxacin. Hence, studying the
interaction of a probiotic with an antibiotic drug is essential prior
to co-administration of the probiotic with the oral antibiotic.
Introduction
Probiotics are live bacterial agents present in the normal gut flora
with low or no virulent activity and which are known to be beneficial
to the health of the host when administered in adequate numbers (Saavedra, 1995; Hozapfel et al., 1998). Bacteria of the genus
Lactobacillus, Lactococcus and Bifidobacterium are
well known probiotics with desirable properties and documented
clinical effects (Salminen et al., 1998). Probiotics have
been widely used in food producing animals such as cattle, pigs and
chickens for growth performance and prevention against pathogenic
microbial infection with and without oral antibiotics.
Lactobacillus acidophilus is a lactic acid producing,
Gram-positive bacteria (Kandler, 1983) which has shown a
variety of pharmacological effects in rats. It is the most
commercially utilized probiotic, especially in the dairy industry (de Vos, 2011).
Probiotics are known to balance the intestinal microbial flora. Their
presence limits the pathogenic potential of another microorganism by
competing for nutrients, lowering the luminal pH, and by producing and
releasing antimicrobial agents; they are also involved in the
modulation of the specific and innate immune system (Shortt, 1998; Mcfarlane and Cummings, 1999; Sanders, 1999;
Oelschlaeger, 2010).
As a result of interacting with the normal microbial flora, probiotics
modulate the composition and activity of the gut enzymes in addition
to providing their own specific enzymatic activities. Hence,
probiotics potentially affect the pharmacokinetics of drugs (Gibson and Roberfroid, 1995). There are some reports of probiotic effects on the
pharmacokinetics of a drug. The effect varies depending upon the
strain of priobiotic bacteria utilized, the host involved, type of
drug used as well as the health status of the animals (Matuskova et al. 2014; Al-Salami et al. 2008; Alvarez-Olmos and
Oberhelman, 2001).
Currently varied research findings have been reported for the effects
of probiotics on the health of their hosts. However, there are only
limited data on the effects of specific probiotics on the
pharmacokinetics of drugs. In addition, there is no information about
the effect of probiotics on the pharmacokinetics and/or
bioavailability of orally administered antibiotics such as
marbofloxacin which has a 100% bioavailability in some animals.
Therefore, the objective of the present work was to investigate the
pharmacokinetics of marbofloxacin administered orally to Sprague
Dawley rats in the absence and presence of L. acidophilus as
a probiotic.
Materials and Methods
Animals
All the procedures with animals in this study were approved by the
institute of animal uses and care committee of Kyungpook National
University (Approval number: KNU-2013-0088). Ten eight-week old
Sprague Dawley (Crl:SD) specific pathogen free (SPF) rats (Orientbio,
Sungnam, Korea) in the same condition were selected. They were
provided with adequate commercial feed 5L79 (PMI Nutrition
International, LLC, Brentwood, MO, USA) and filtered tap water. The
rats were arranged in two groups, consisting of five males and five
females, and were conditioned to the laboratory situation for one
week. Rats in each group were arranged in two cages (25 cm width by 40
cm length by 20 cm height). Each of the cages contained two or three
rats and was provided with sterile 100% virgin wood fiber bedding
(Beta chip®, Northeastern Products Corp. NY, USA). The average body
weights of the female and male rats were 201.2 and 285.2 g,
respectively. Rats were housed at an average temperature of 25
°C and humidity of 60%. The general health status of the rats was
monitored by physical and physiological examination prior, during and
at the end of the study. The room had an equal 12 h of light and
darkness.
Experimental protocol and measurements
On the first and the ninth day of the experiment, rats were given 20 mg/kg of marbofloxacin intragastrically using a 22 G ball tip needle. Starting on the second day, 109 CFU/ml of L. acidophilus KCTC 3140 (obtained from the Korean collection for type culture, Daejon, South Korea) (Park et al., 2006) in 200 µl volume was administered intragastrically daily for 7 days. A maximum of 300 µl of blood was collected using a microvette (Microvette ® CB 300 K2E, Sarstedt, Germany) from the tail of rats at 0, 0.25, 0.5, 0.75, 1, 2, 4, 8 and 12 h after administration of marbofloxacin. Finally, the plasma was collected after centrifugation at 10000 RPM for 10 min at 4 °C and stored at -20 °C until analyzed using high performance liquid chromatography (HPLC).
The marbofloxacin concentration in the plasma was
measured by HPLC using a Hewlett Packard Agilent 1100 series
comprising an HPLC pump, HP ODS Hypersil column (250 × 4.6mm, 5µm),
autoinjector and UV detector. The wavelength of the UV detector was
set at 293 nm and the column temperature was 30 °C. An aliquot of rat plasma was deproteinated by adding an equal
amount of acetonitrile. After vortex-mixing and centrifugation at
16,000 × g for 1 min, a 20 μl aliquot of the
supernatant was added to the auto-sampler vial and injected directly
onto the HPLC column. The mobile phase consisting of 10% acetonitrile,
10% methanol and 80%, 20 mmol potassium phosphate (0.05 M, ACS
reagent, Sigma® ≥99.0% purity, pH=2.9) buffer, pH =3 was run at a flow
rate of 1 ml/min.
The standard and quality control (QC) samples were prepared using
stock solutions of marbofloxacin (1 mg/ml; Fluka, Sigma-Aldrich,
Germany). Drug free rat plasma samples were spiked with these
solutions to prepare standard curves, determine the accuracy,
precision and detection limits of the assays. The same samples were
also used as QC samples for intra-assay and inter-assays. All the
stock solutions were stored at 4 °C until used.
Method validation was determined according to the FDA guidelines. The
detection limit and quantitation limit were calculated based on the
standard deviation of the response and the slope of the calibration
curve.
The calibration curve was created using a high concentration of
marbofloxacin (100 µg/ml) in plasma and stepwise dilutions of 50, 20,
10, 5, 2, 1, 0.5, 0.25, 0.1, 0.05, 0.02, and 0.01 µg/ml. Analyses were
based on peak areas. Three sets of calibration curves were used to
validate the method. The data were validated using a standard
statistical curve. The intra-assay precision was determined at 20, 4,
0.5 and 0.05 µg/ml. Accuracy was determined by comparing the measured
concentrations with the calculated nominal concentrations.
A bioassay of the plasma was performed to standardize the HPLC result.
Ten ml of LB agar (DifcoTM, BD, USA) was added to a
sterile plate. E. coli strain BE was grown in LB broth and
transferred to sterilized LB agar media at a specific dilution rate.
The LB agar media containing 106-107 CFU/ml of the bacteria was added
to the provided LB agar plate. Then the media were kept at 4
°C until used.
A paper disc was sterilized by autoclaving and 60 µl of plasma was
applied in a biological safety cabinet. After the paper had been
dried, it was transferred to the prepared agar plate and incubated
overnight at 37 °C aerobically. A known concentration of marbofloxacin in distilled
water and in plasma, as well as plasma alone, were used as controls
for HPLC analysis.
Statistical analysis
The statistical analysis was conducted using SAS version 9.4 (SAS
Institute Inc., NC, USA). The Phoenix WinNonlin (Pharsight Corp., St.
Louis, MO, USA) software program was used to compute the
pharmacokinetics analysis. A trapezoidal method of non-compartmental
analysis was used for each plasma concentration and the data were
analyzed using nonlinear least-squares regression analysis. Comparison
of the mean values of the pharmacokinetic parameters before and after
treatment was statistically evaluated using t-test and the P value
<0.05 was considered as statistically significant.
Table 1. Pharmacokinetics of MRB (Mean + SE) before and after treatment with L. acidophilus
|
Parameters |
Units |
Before |
After |
AUC |
µg.h/ml |
5.78 + 0.56 |
2.57 + 0.13* |
K01_HL |
H |
0.17 + 0.03 |
0.39 + 0.1* |
K10_HL |
H |
1.19 + 0.2 |
0.69 + 0.18* |
CL_F |
ml/h/mg |
699.55 + 69.19 |
1560.83 + 81.31* |
Tmax |
H |
0.54 + 0.06 |
0.73 + 0.03 |
Cmax |
µg /ml |
2.42 + 0.08 |
1.24 + 0.02* |
AUC, area under the concentration-time curve;
K01_HL, Half-life of absorption;
K10_HL, Elimination half-life;
CL_F, total body clearance;
Tmax, Time taken to achieve maximum concentration;
Cmax, maximum concentration.
*Statistically significant difference at P<0.05.
Results
Method Calibration
The HPLC retention time for marbofloxacin was 7.8 min at a flow rate
of 1 ml/min. The spiked samples showed peaks without any interference
at the specified retention time (Fig 1). A linear relationship was
maintained for the calibration curve at both lower and higher
concentrations. The linearity of the standard curve of marbofloxacin
concentration in the spiked plasma was shown by the value of
regression (R2=0.9986).
Marbofloxacin was detected at a concentration of 0.01 µg/ml. Hence,
the limit of detection (LOD) was 0.01 µg/ml and the limit of
quantitation (LOQ) was 0.05 µg/ml. The inter-day and intra-day
coefficients of variation were < 10. The overall bias of the plasma
sample was 6.5%.
Pharmacokinetics
The calculated PK parameters of marbofloxacin in plasma before and
after treatment of the rats with L. acidophilus are
summarized in Table 1. The kinetics of marbofloxacin are best
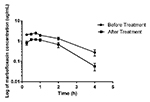
described by a one-compartment open model. The area under the curve
(AUC) of marbofloxacin was significantly decreased from 5.78 µg.h/ml
to 2.56 µg.h/ml after the rats were treated with
L. acidophilus (Fig 2). Likewise, the Cmax declined from 2.4
µg/ml to 1.2 µg/ml and the Tmax increased from 0.54 to 0.73 h. In this
study, no significant difference was observed between the two sexes.
The elimination half-lives of marbofloxacin before and after treatment
with L. acidophilus were 1.19 h and 0.69 h, respectively.
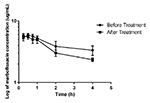
The linearity of the spiked plasma in the bioassay showed a
logarithmic correlation of R² = 0.9868. The microbiological assay
result showed that the maximum concentration of marbofloxacin was 7.66
µg /ml and 7.08 µg/ml which were obtained at 0.5 h and 0.25 h before and
after treatment, respectively (Fig 3).
Discussion
The utilization of probiotics in human health in the recent years has become more popular. Lactic acid producing bacteria including, Lactobacillus, Lactococcus and Bifilidobacterium are the most widely commersialised probiotic bacteria (Vasiljevic and Shah, 2008; Holzapfel et al., 2001). There is increasing scientific evidence that consumption of probiotics in adequate numbers confers health benefits to humans and animals (Kechagia et al., 2013; Narayan et al., 2010). These bacteria are used as an antimicrobial agent but they will also metabolise antibiotics and can be used in the treatment of acute diarrheal diseases, prevention of antibiotic-associated diarrhea, and improvement of lactose metabolism (Kechagia et al., 2013; Wilson and Nicholson, 2009). Furthermore, there are recent reports of the effects of probiotics on the pharmacokinetics of antimicrobial agents. However, the results vary depending on the type probiotic strains applied, the drug tested and the type of study conducted.
In this particular study, we have tried to establish the effect of
L. acidophilus on the pharmacokinetics of marbofloxacin using
a rat model. It has been shown that the plasma concentration of
marbofloxacin was reduced in the rats treated with
L. acidophilus for 7 days, indicating that the probiotic
decreases the bioavailability of the antibacterial agent. This result,
agrees with the reports of Al-Salami and his colleagues (2008), who
showed a decreased concentration of gliclazide in healthy rats after
treatment with three different probiotics. The probiotic treatment
reduced gliclazide absorption and bioavailability in healthy rats.
This might be attributed to the activation of the intestinal efflux
drug transporter by the probiotics. Another explanation might be the
formation of a ‘thicker’ layer of the
adherent mucous, which comprises the physical barrier protecting the
enterocytes. In addition, the probiotic might stimulate the
presystemic metabolism of the drug (Al-Salami et al., 2008; AlSalami et al., 2012a).
However, the result is contrary to the findings of Matuskova
et al. (2014), who reported the increased
bioavailability of amidarone and altered pharmacokinetics of the drug
after utilizing E. coli Nissle 1917 as a probiotic. This
difference in response might be due to the application of different
strains of probiotics with different drugs.
Probiotics affect the metabolism of drugs in the gut. They induce
various cytochrome enzymes, or phase II conjugating enzymes, in the
intestine responsible for drug metabolism while others like,
L. acidophilus, upregulate intestinal electrolyte absorption
while inhibiting the cellular uptake of micellar cholesterol (Wilson and Nicholson, 2009; Raheja et al., 2010; Huang and Zheng,
2010). L. helveticus R389 indirectly affects calcium metabolism
through enhanced expression of the main calcium transporter in the
epithelial cells of the duodenum (Vinderola et al., 2007; Resta, 2009). The alteration of drug bioavailability might also be affected by
the expression of intestinal transporters that are involved in drug
transport or by decreasing the expression of proteins which are
important for the disposition of drugs (Saksena et al., 2011; Matuskova et al., 2011; Huang et al.,
2010).
In conclusion, the treatment of rats with L. acidophilus in
the present study significantly decreased the plasma AUC and Cmax, but
increased the clearance of orally administered marbofloxacin. The type
of effect exerted by a certain probiotic strain depends on its
metabolic properties, the kind of surface molecules expressed or on
the secreted components. Hence, strain identification is recommended
to establish probiotic suitability and performance for commercial
application since, closely related probiotic strains may have
different clinical effects (Alvarez-Olmos and Oberhelman, 2001). This can be achieved by a combination of phenotypic and genetic
identification tests (FAO, 2001). In addition, prior to
administration of probiotics along with antibiotics,
in vivo studies should be carried out instead of relying only
on in vitro experiments, and the bioavailability of oral
antibiotics should be determined. Interestingly, although there are
clear differences in the pharmacokinetic effects of probiotics (Al-Salami et al., 2008; Al-Salami et al., 2012a; Matuskova et al.,
2014), the relationships between pharmacokinetic changes and intestinal
microbiota have not been studied. Therefore, we plan to conduct
genetic studies to determine the relationship between marbofloxacin
pharmacokinetics and metagenomics of intestinal microbiota.
Acknowledgements
This work was in part supported by a grant the Technology Development Program for Forestry (S111515L050130), Korea forest service, in part by the Technology Commercialization Support Program (314082-3), Ministry of Agriculture, Food and Rural Affairs, and in part by Cooperative Research Program for Agriculture Science & Technology Development (PJ01128901), Rural Development Administration.
References
-
Al-Salami H, G Butt, I Tucker, R Skrbic, S Golocorbin-Kon & M Mikov: Probiotic pre-treatment reduces gliclazide
permeation (ex vivo) in healthy rats but increases it in
diabetic rats to the level seen in untreated healthy rats. Arch.
Drug Inf. 2008, 1(1), 35–41.
-
Al-Salami H, G Butt, I Tucker, S Golocorbin-Kon, & M
Mikov: Probiotics decreased the bioavailability of the bile acid analog,
monoketocholic acid, when coadministered with gliclazide, in healthy
but not diabetic rats. Eur. J. Drug Metab. Pharmacokinet. 2012a,
37, 99-108.
-
Alvarez-Olmos MI & AR Oberhelman: Probiotic agents and
infectious diseases: A modern perspective on a traditional therapy.
Clin. Infect. Dis. 2001, 32(11),
1567-1576.
de Vos WM: Systems solutions by lactic acid bacteria: from paradigms to practice. Microb. Cell Fac. 2011, 10(Suppl 1), S2.
-
FAO/WHO, Report on Joint FAO/WHO Expert Consultation on
Evaluation of Health and Nutritional Properties of Probiotics in
Food Including Powder Milk with Live Lactic Acid Bacteria, 2001.
-
Gibson GR & MB Roberfroid: Dietary modulation of the
human colonic microbiota: introducing the concept of prebiotics. J
Nutr. 1995, 125, 1401-1412.
-
Holzapfel WH, P Haberer, R Geisen, J Björkroth, & U
Schillinger: Taxonomy and important features of probiotic microorganisms in
food and nutrition. Am. J. Clin. Nutr. 2001, 73(2),
365S–373S.
-
Hozapfel WH, P Haberer, J Snel, U Schillinger & JHJ Veld: Overview of gut flora and probiotics. Int. J. Food Microbiol. 1998,
41, 85-101.
-
Huang Y & Y Zheng: The probiotic Lactobacillus
acidophilus reduces cholesterol absorption through the
down-regulation of Niemann-Pick C1-like 1 in Caco-2 cells. Br. J.
Nutr. 2010, 103, 473-478.
-
Huang Y, J Wang, Y Cheng & Y Zheng: The
hypocholesterolaemic effects of Lactobacillus acidophilus American
type culture collection 4356 in rats are mediated by the
down-regulation of Niemann-Pick C1-like 1. Br. J. Nutr. 2010,
104, 807-812.
-
Kandler O: Carbohydrate metabolism in lactic acid
bacteria. Antonie van Leeuwenhoek. 1983, 49,
209–224.
-
Kechagia M, D Basoulis, S Konstantopoulou, D
Dimitriadi, K Gyftopoulou, N Skarmoutsou, & EM
Fakiri: Health Benefits of Probiotics: A Review. Int. Sch. Res. Notices. 2013, 2013, 1-7.
-
Matuskova Z, E Anzenbacherova, R Vecera, H Tlaskalova-Hogenova, M
Kolar & P Anzenbacher:
Administration of a probiotic can change drug pharmacokinetics:
Effect of E. coli Nissle 1917 on amidarone absorption in
rats. PLoS ONE. 2014, 9(2), e87150.
-
Matuskova Z, M Siller, A Tunkova, E Anzenbacherova, A Zacharova,
H Tlaskalova-Hogenova, Z Zidek & P Anzenbacher:
Effects of Lactobacillus casei on the expression and the activity of
cytochromes P450 and on the CYP mRNA level in the intestine and the
liver of male rats. Neuro Endocrinol. Lett. 2011,
32, 8-14.
-
McfarlaneG & JH Cummings: Probiotics
and prebiotics: can regulating the activities of intestinal bacteria
benefit health? BMJ 1999, 318, 999-1003.
-
Narayan SS, S Jalgaonkar, S Shahani & VN Kulkarni:
Probiotics: current trends in the treatment of diarrhea. Hong Kong
Med. J. 2010, 16, 213-218.
-
Oelschlaeger TA. Mechanisms of probiotic actions - A
review. Int. J. Med. Microbiol. 2010, 300, 57-62.
-
Park SC, MH Hwang, YH Kim, JC Kim, JC Song, KW Lee, KS Jeong, MH Rhee, KS Kim & TW Kim: Comparison of pH and
bile resistance of Lactobacillus acidophilus strains
isolated from rat, pig, chicken, and human sources. World J.
Microbiol. Biotechnol. 2006, 22 (1),
35-37 .
- Raheja G, V Singh, K Ma, R Boumendjel, A Borthakur, RK Gill, S Saksena, WA Alrefai, K Ramaswamy, & PK Dudeja:
-
Lactobacillus acidophilus stimulates the expression of
SLC26A3 via a transcriptional mechanism. Am. J. Physiol.
Gastrointest. Liver Physiol. 2010, 298(3), G395–G401.
-
Resta SC: Effects of probiotics and commensals on
intestinal epithelial physiology: implications for nutrient
handling. J. Physiol. 2009, 587, 4169-4174.
-
SaavedraJM: Microbes to fight microbes: a not so
novel approach to controlling diarrheal disease. J. Pediatr.
Gastroenterol. Nutr. 1995, 21, 125-129.
-
Saksena S, S Goyal, G Raheja, V Singh, M Akhtar, TM Nazir, WA
Alrefai, RK Gill & PK Dudeja: Upregulation of P-glycoprotein by probiotics in intestinal
epithelial cells and in the dextran sulfate sodium model of colitis
in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2011,
300, G1115–G1123.
-
Salminen S, C Bouley, MC Boutron-Rualt, JH
Cummings, A Franck, GR
Gibson, E Isolauri, MC
Moreau, M
Roberfroid & I Rowland: Functional food science and
gastrointestinal physiology and function. Br. J. Nutr. 1998,
80(Suppl 1), S147-S71.
-
SandersME: Probiotics. Food. Technol.
1999,53,67-77.
-
ShorttC: Living it up for dinner. Chem. Ind. 1998,
8, 300-303.
-
Vasiljevic T & NP Shah. Probiotics from Metchnikoff to
bioactives. Int. Dairy J. 2008, 18, 714-728.
-
Vinderola G, C Matar, & G Perdigón: Milk fermentation
products of L. helveticus R389 activate calcineurin as a
signal to promote gut mucosal immunity. BMC Immunol. 2007,
8(19), 1-10.
- Wilson ID & JK Nicholson: The role of gut microbiota in drug response. Curr. Pharm. Des. 2009, 15, 1519-1523.