Technical report
PET radioligand injection for pig neuroimaging
by Aage KO Alstrup1, Ole L Munk1, Anne M Landau1,2 and Thea P Lillethorup1
1Department of Nuclear Medicine & PET center
2Translational Neuropsychiatry Unit, Aarhus University and Hospital,
Denmark
 Correspondence: Aage Kristian Olsen Alstrup, DVM & PhD
Correspondence: Aage Kristian Olsen Alstrup, DVM & PhD
Department of Nuclear Medicine & PET center
Nørrebrogade 44, 10C
DK, 8000-Aarhus C, Denmark.
Email: aagols@rm.dk
Summary
Pigs are useful models in neuroimaging studies with positron emission tomography (PET). Radiolabeled ligands are injected intravenously at the start of the scan and in pigs the most easily accessible route of administration is the ear vein. However, in brain studies the short distance between the brain and ear vein can be problematic as both are localized inside the field of view and, as a consequence, tracer residues in the catheter may influence the outcome of the scan. Here, we discuss options to avoid this problem. The femoral vein can be used in studies where repeated arterial blood sampling is needed because surgical incision has to be performed to allow access to the artery. When a non-invasive technique is preferred, the ear vein is a good alternative although it is recommended to dilute the tracer sufficiently in saline (20-50 mL) prior to injection. In addition, the tracer can be injected through an extension tube (filled with saline before injection), which is removed together with the syringe immediately after tracer injection. This avoids placing the syringe with tracer inside the PET gantry while injecting. By applying these simple techniques, it is our experience that it is possible to obtain high-quality images without exposing pigs to invasive procedures.
Introduction
Positron emission tomography (PET) is a technique for the study of physiological and pathophysiological processes in the living organism (Bailey et al., 2005). Along with other imaging modalities, PET is a useful tool to gain insight into the in vivo functions of the healthy and diseased brain. For this purpose, the pig is a suitable animal model as it has a relatively large gyrencephalic brain with high resemblance to the human brain, which is an advantage due to the low spatial resolution (3-5 mm FWHM) with PET (Alstrup & Smith, 2012). Furthermore, the translational value is strengthened by use of human scanners. In PET, a tracer is administered intravenously, after which the scanner measures the emitted photons and images of the tracer distribution in the brain can be reconstructed (Bailey et al., 2005). The vein that superficially gives the easiest accessibility in both minipigs and domestic pigs is the ear vein, and it is generally used in pre-clinical studies (Alstrup, 2010; Bollen et al., 2010; Gillings et al., 2001; Landau et al., 2017). However, in brain studies the short distance between the brain and ear vein can be problematic, as both are localized inside the field of view, and as a consequence tracer residues in the catheter may influence the outcome of the scan. Although only a very small volume of the tracer adheres to the catheter, such a highly concentrated point source can affect the quality of the image as it increases the incidence of local random scatter. This might result in so-called streaking or halo artifacts in the images, as is also seen in areas adjacent to structures with high tracer uptake (i.e. bladder or kidneys) (Noto et al., 2017).
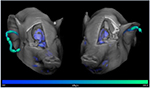
Figure 1 shows how tracer residues may appear in [11C]PK11195 PET
images. [11C]PK11195 PET is commonly used for detection of
neuroinflammation in humans as it binds to the translocator protein 18
kDa (TSPO), which is highly expressed in activated microglia. It has
also previously been used in pig imaging studies (Cumming et al., 2001; 2006). Our group recently embarked on studies of neurodegenerative
disease in pigs with the [11C]PK11195 PET tracer (data not published).
In these studies, although the same amount (300-400 MBq) and volume
(10 mL) of tracer was used, the amount of tracer retained in the ear
vein differed between individual minipigs. Similar problems of tracer
sticking to the internal tubing of catheters have been seen with other
tracers. Here we will discuss various methods to avoid or reduce this
problem based on our studies with different tracers.
|
Figure 1. Click image to enlarge |
Materials, methods and results
Animals
Our investigations are reported in compliance with the ARRIVE
guidelines. All studies were performed on adult female Göttingen
minipigs from Ellegaard Minipigs ApS (Dalmose, Denmark) on licences
(2016-15-0201-00878 and 2016-15-0201-01138) issued by The Danish
Experimental Animal Inspectorate. Minipigs were fed a restricted
pellet diet (SDS, Aarhus, Denmark) and fasted overnight prior to
studies, with continuous access to tap water. The minipigs were used
in different brain research projects (data yet not published), and
they were acclimated for several weeks at 20oC and 50-55 % relative
humidity prior the studies, with a controlled photo-period of 12 hours
light and with air changed eight times every hour. Minipigs were
single or group housed in 4.6 m2 enclosures with fence-line contact
with congeners.
Dilution of tracer prior to injection
Dilution of the tracer prior to injection and flushing with saline
after injection are obvious possible options for reducing the residue
in the catheter. We have examined the effect of tracer volume and
flushing volume in an in vitro experiment. We used
[18F]FDOPA, a marker of dopamine synthesis widely used in pig brain
studies (Danielsen et al., 2001) which we also have
previously observed to adhere to the ear catheters. We injected
approximately 100 MBq tracer in 22G catheters (BD Venflon ProSafety
0.9mm x 25 mm; Abena, Aabenraa, Denmark), which initially had been
flushed with saline. The tracer was diluted in saline to total volumes
of 5 mL, 10 mL, 20 mL and 50 mL. Similarly, the catheters were
immediately flushed with 5 mL, 10 mL, 20 mL or 50 mL of saline after
tracer injection. Subsequently, the residue of the tracer was measured
(one minute/sample) in a gamma counter (Packard Cobra Gamma Counter,
Model D5003, GMI Inc, Ramsey, Minnesota, USA), and the residues were
calculated as % of the injected tracer dose. The experiment was
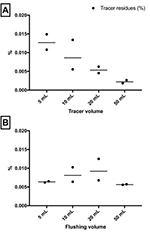
repeated and gave similar results both times (Figure 2). We found a
large effect of diluting the tracer prior to injection (from
0.011-0.015% in 5 mL to 0.002-0.003% in 50 mL volume, Figure 2A),
while the flushing volume (5 mL - 50 mL, Figure 2B) had no effect.
This suggests that diluting the tracer in a larger volume will result
in a smaller portion of the tracer coming into contact with the
surface of the catheter and adhering to the plastic surface. However,
once the tracer has adhered to the surface it is difficult to flush
away with saline, and therefore the flushing volume had no effect in
this setup. Consequently, it is unlikely that a saline drip will have
a significant impact on reducing catheter-bound tracer residues.
Based on these results, we first increased the tracer volumes from 10 mL to 20 mL or 50 mL in our ongoing minipig studies (data not published). In one study, we found no visible residues in ear catheters when using 50 mL volume of [18F]fludeoxyglucose (6 minipigs scanned 3 times = 18 scans), a widely used tracer for the study of glucose metabolism in the brain (Poulsen et al., 1997). The disadvantage of increasing the injected tracer volume to such a large extent is that this increases the injection time and thereby compromises injection of the tracer as a true bolus. Moreover, even though the tracer is diluted in a larger volume, streaking artifacts still occurred during the injection and therefore an investigator should strive for a minimal injection time. The extent to which this is important depends on the purpose of the study, the nature of the tracer and the plans for data quantification and kinetic modeling. Therefore, there is a trade-off between having a fast injection and increasing the injection volume to reduce ear vein residues.
|
Figure 2. Click image to enlarge |
Removing the catheter immediately after injection
Another option is to remove the catheter immediately after the
injection of particularly sticky tracers. We have tried this method in
an experiment with [11C]PIB, a tracer for detection of beta-amyloid in
the brain (Laforce et al., 2011). By removing the catheter
immediately after injection, we limited the radiation from the ear
during the first minute of the scan. This corresponds to the time it
takes to inject the tracer and flush with saline. Furthermore, it has
allowed us to measure how much tracer remains in the catheter and not
just in the syringe. We have thus found that 0.2% - 0.6% of the
injected dose was left in the ear vein catheter.
Although the percentage of dose remaining in the catheter is small,
it is present in a much smaller volume than the injected
dose (i.e. 0.2 mL catheter volume vs 20 L pig volume).
Therefore the activity concentration of the tracer remaining in the catheter will be substantial. The disadvantage of removing the ear vein catheter is that it will only be possible to inject one tracer. Therefore, it would limit the possibility to conduct multi-tracer studies. A way to circumvent this issue would be to insert one catheter in each ear vein for multi-tracer studies. Another option, if multi-tracer studies are planned, would be to first inject a tracer that is not sticky (for example, raclopride) prior to injection of the neuroinflammation or protein binding markers that adhere to the catheters. It is important to note that removing the catheter during the first 1-2 minutes of the scan will affect the attenuation scan, and therefore we recommend the attenuation maps to be acquired after the PET scan and the initial minute of the scan to be excluded from the kinetic analysis. Finally, if bleeding is not stopped immediately after ear vein catheter removal, blood-containing tracers can contaminate the skin and so would require attention from a radiation safety viewpoint.
Other injection sites
Instead of using the ear vein for tracer injections, it is possible to
catheterize other veins in the pigs. When blood sampling is needed for
kinetic analysis, a catheter is normally placed in the femoral artery.
As the femoral artery and vein are located close to each other, they
can easily be catheterized together with only minor changes to the
surgical procedure (Ettrup et al., 2011). A further advantage
is that the injection would take place outside the field of view of
the PET cameras. The disadvantage is that a femoral vein
catheterization requires surgical intervention or ultrasonic guided
techniques, which also affects the welfare of the animal. Furthermore,
the pig is usually placed on its back in order to gain access to the
catheter, which in long-term studies may affect lung function. Our
experience is also that scar tissue formation is substantial following
a catheterization in the femoral artery, which complicates using the
same site twice for injection in longitudinal studies. Other
alternatives for accessible veins in the minipig include the
peripheral milk vein (Ettrup et al., 2010; Andersen et al., 2015), the cephalic vein or the saphenous veins, but we have not yet used
them as injection sites for PET neuroimaging studies.
Image reconstruction
As already mentioned, the artifacts observed in or close to the brain
following ear-vein injection were similar to halo or streaking
artifacts seen in areas close the bladder and the kidneys, which tend
to accumulate high amount of tracer during a scan. This artificially
reduced apparent tracer uptake was probably related to scatter and
attenuation correction and might be restored partly by disabling or
scaling the scatter correction. However, non-scatter corrected PET
images are not quantitative. Reducing the dead time of the detector
ring or optimizing the acquisition time could further improve image
quality (Lütje et al., 2016), but investigating different
image reconstruction possibilities was outside the scope of this
paper.
Discussion
Large animal models including pigs are important for translational neuroscience, and PET imaging enables a unique insight into the functions of the healthy and diseased brain. For PET tracer injection, the femoral vein is preferable to the ear vein. Conveniently, the femoral vein can be used in studies where repeated arterial blood sampling is needed, as surgical incision is performed to allow access to the artery. When a non-invasive technique is preferred, the ear vein is a good alternative although it is recommended to dilute the tracer sufficiently prior to injection. As an example, the tracer [18F]FDOPA requires a volume of 20 mL or more to reduce tracer residues, and based on our practical experience with pigs this volume will also help when using other PET tracers. As most tracers used for dynamic PET scanning should be given as bolus injections (< 1 minute), volumes larger than 50 mL are impractical. Moreover, according to the scientific recommendations, a 20 kg pig should as a maximum have a 50 mL intravenous bolus injection (Diehl et al., 2001). The tracer can furthermore be injected through an extension tube (filled with saline before injection), which is removed together with the syringe after tracer injection. This avoids placing the syringe with tracer inside the PET gantry while injecting. The ear of the pig can be secured with tape towards the back along the neck in order to maximum the distance from the brain. Securing the ear has the additional advantage that it cannot move during the injection, which decreases the attenuation correction error in the initial frames of the PET images if the catheter is not removed immediately after injection. By applying these simple techniques it has been, in our experience, possible to obtain high-quality images without exposing pigs to invasive procedures.
Conclusion
We have found that injection of certain PET tracers into the ear vein of pigs can lead to scanning artifacts. In this paper, we suggest measures to reduce these effects. We discuss the benefits of increasing the injection volume, removing the catheter immediately after injection and changing the tracer injection site. Each of these solutions can lead to other study limitations, so a fine balance is required depending on the type of tracer and the purpose of the study (single tracer vs multi-tracer, acute vs. longitudinal, etc).
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding
This study was financially supported by Aarhus University PhD funding for TPL, and grants from the Lundbeck Foundation (grant number 2013-16034) and the Bjarne Saxhof Fund, administered through the Danish Parkinson’s Foundation, to AML.
References
- Alstrup AKO & DF Smith: PET neuroimaging in pigs. Scand. J. Lab. Anim. Sci. 2012, 39(1), 25-45.
- Alstrup AKO: Anesthesia and analgesia in Ellegaard Göttingen minipigs. Ellegaard, Dalmose, DK, 2010.
- Andersen VL, HD Hansen, MM Herth, A Dyssegaard, GM Knudsen & JL Kristensen: 11C-labeling and preliminary evaluation of pimavanserin as a 5-HT2A receptor PET-radioligand. Bioorg. Med. Chem. Lett. 2015, 25, 1053-1056.
- Bailey DL, DW Townsend, PE Valk & MN Maisey: Positron Emission Tomography: Basic Sciences, Springer-Verlag, London, UK, 2005.
- Bollen PJA, AK Hansen & AKO Alstrup: The laboratory swine. CRC Press, Boca Raton, Fl. 2010.
- Cumming P, EH Danielsen, M Vafaee, L Falborg, E Steffensen, JC Sorensen, N Gillings, D Bender, K Marthi, F Andersen, O Munk, D Smith, A Moller & A Gjedde: Normalization of markers for dopamine innervation in striatum of MPTP lesioned miniature pigs with intrastriatal grafts. Acta. Neurol. Scand. 2001, 103, 309-315.
- Cumming P, MD Pedersen, L Minuzzi, K Mezzomo, EH Danielsen, P Iversen, D Aagaard, S Keiding, OL Munk & B Finsen: Distribution of PK11195 binding sites in porcine brain studied by autoradiography in vitro and by positron emission tomography. Synapse 2006, 59, 418-426.
- Danielsen EH, DF Smith, F Andersen, AD Gee, D Bender, SB Hansen, F Hermansen, L Ostergaard, P Cumming & A Gjedde: FDOPA metabolism in the adult porcine brain: influence of tracer circulation time and VOI selection on estimates of striatal DOPA decarboxylation. J. Neurosci. Methods. 2001, 111, 157-168.
- Diehl K-H, R Hull, R Morton, R Pfister, Y Rabemampianina, D Smith, JM Vidal & C van de Vorstenbosch: A good practice guide to the administration of substances and removal of blood, including routes and volumes. J. Appl. Toxicol. 2001, 21, 15-23.
- Ettrup A, M Palner, N Gillings, MA Santini, M Hansen, BR Kornum, LK Rasmussen, K Någren, J Madsen, M Begtrup & GM Knudsen: Radiosynthesis and Evaluation of 11C-CIMBI-5 as a 5-HT2A Receptor Agonist Radioligand for PET. J. Nucl. Med. 2010, 51, 1763-1770, DOI: 10.2967/jnumed.109.074021.
- Ettrup KS, AN Glud, D Orlowski, LM Fitting, K Meier, JC Sørensen, C Bjarkam & AKO Alstrup: Basic surgical techniques in the Göttingen minipig: intubation, bladder catheterization, femoral vessel catheterization, and transcardial perfusion. J. Vis. Exp. 2011, 52, 2652. DOI: 10.3791/2652.
- Gillings NM, D Bender, L Falborg, K Marthi, OL Munk & P Cumming: Kinetics of the metabolism of four PET radioligands in living minipigs. Nucl. Med. Biol. 2001, 28, 97-104.
- Laforce R & GD Rabinovici: Amyloid imaging in the differential diagnosis of dementia. Review and potential clinical applications. Alzheimers Res. Ther. 2011, 3(6), 31. DOI: 10.1186/alzrt93.
- Landau AM, AKO Alstrup, H Audrain, S Jakobsen, M Simonsen, A Møller, P Videbech, G Wegener, A Gjedde & DJ Doudet: 2 J. Cereb. Blood Flow Metab. 2017, Jan 1, 271678X17705260. DOI: 10.1177/0271678X17705260.
- Lütje S, S Blex, B Gomez, BM Schaarschmidt, L Umutlu, M Forsting, W Jentzen, A Bockisch, TD Poeppel & A Wetter: Optimization of Acquisition time of 68Ga-PSMA-Ligand PET/MR in Patients with Local and Metastatic Prostate Cancer. PLoS One. 2016, 11(10), e0164392. DOI: 10.1371/journal.pone.0164392.
- Noto B, F Büther, KA Springe, N Avramovic, W Heindel, M Schäfers, T Allkemper & L Stegger: Impact of PET acquisition durations on image quality and lesions detectability in whole-body 68Ga-PSMA PET-MRI. EJNMMI. Research. 2017, 7(12), DOI: 10.1186/s13550-017-0261-8.
-
Poulsen PH, DF Smith, L Ostergaard, EH Danielsen, A Gee, SB
Hansen, J Astrup & A Gjedde:
In vivo estimation of cerebral blood flow, oxygen consumption and
glucose metabolism in the pig by [15O]water injection, [15O]oxygen
inhalation and dual injections of [18F]fluorodeoxyglucose. J.
Neurosci. Methods. 1997, 77, 199-209.