Original scientific article
Effects of exogenous sex hormones on mouse estrous cycle, vaginal microbiota and immune cells
by Priscilla Romina De Gregorio, Susana Salva, María Silvina Juárez Tomás, María Elena Fátima Nader-Macías
Centro de Referencia para Lactobacilos (CERELA)-CONICET, Chacabuco
145, 4000, San Miguel de Tucumán, Tucumán, Argentina
 Correspondence: María Elena Nader-Macías
Correspondence: María Elena Nader-Macías
CERELA-CONICET
Chacabuco 145. San Miguel de Tucumán
(T4000ILC). Tucumán. Argentina
Phone/ Fax: +54-381-4311720/4310465
E-mail: fnader@cerela.org.ar
Summary
Sex hormones are often administered to mice in vaginal microbial studies in order to synchronize estrous. Our objective was to evaluate the effect of estradiol-hemisuccinate (EH) or medroxyprogesterone-acetate (MPA) administration on estrous cycle, vaginal microbiota, and immune cell populations of various organs in a murine model. Two-month-old female BALB/c mice were intramuscularly injected with EH (day -2) or MPA (day -5) to induce estrous (E) or diestrous (DE), respectively. On sampling days (Sd) 0, 2, 6 and 8, vaginal washings (v.w.), vagina, blood, spleen and bone marrow (BM) samples were taken. Most of the animals remained in E or DE states until Sd 6 after EH or MPA administration, respectively. The number of cultivable vaginal bacteria was not modified by hormonal treatments; higher quantities were detected in mice in E. Only EH administration modified serum sex hormone levels, increasing serum estradiol on Sd 0. In v.w., myeloid population was dominant while lymphoid populations were not detected. Only MPA administration induced a reduction in myeloid cells on Sd 0. Hormonal treatments did not affect myeloid populations in BM but caused a slight decrease in T and B cells. In spleen, hormonal administration did not affect B or T population size while an increase in mature B cells and a decrease in immature B cells were observed in MPA-treated mice compared with EH-treated mice. Thus, although both hormonal treatments induced slight changes in some of the parameters evaluated compared to control animals, adequately standardized and consistent experimental protocols were established for further studies.
Introduction
The female reproductive tract is a specialized and highly dynamic organ system that is affected by numerous sequential physiological and morphological changes driven by cyclical fluctuations of reproductive hormones (Wira et al., 2010). Hormones exert their effect on various target sites. One of these sites is the vaginal epithelium, a constant cell renewal system in which the cells divide and mature from the basal membrane and are released into the lumen and then into the vaginal canal. Cell maturation, surface layer thickness and desquamation are dependent on estrogen stimulation (Larsen, 1993). Moreover, hormonal fluctuations also exert their effect on the microbial colonization of the vaginal mucosa. It has been suggested that the composition of the vaginal microbiome is influenced by estrogens, based on research demonstrating that these hormones stimulate the deposition of glycogen in the vaginal epithelial tissue, which could be metabolized by native vaginal microorganisms (Larsen, 1993; Eschenbach et al., 2000).
In addition, the female reproductive tract has a very specialized
immune system which has evolved to afford protection against
pathogenic agents. This is possible due to the action of sex hormones
that regulate different immunological parameters such as transport of
immunoglobulins, cytokine levels, distribution of different cell
populations and antigen presentation in genital tissues during the
reproductive cycle (Wira et al., 2010). Furthermore, sex hormones
exert their effect at the systemic level on a wide cell variety
(Sakiani et al., 2013).
Mice are the mammals of choice for many types of
in vivo experimentation because of their small size and short
reproductive cycle. Their estrous cycle lasts 4-5 days and is divided
into 4 phases: proestrous (estimated time, 18 h), estrous (~28 h),
metaestrous (~8 h), and diestrous (~53 h) (Byers et al., 2012; Mclean
et al., 2012). The murine vaginal tract is lined by a keratinizing
stratified squamous epithelium. The vaginal epithelium is
characterized by the presence of keratin in proestrous and estrous
states, leucocyte influx at metaestrous and a thin epithelium at
diestrous (OECD, 2009). Experimental estrogen or progesterone
treatments in mice, in established protocols, produce states where the
vaginal epithelium remains keratinized or extremely thin, respectively
(Kaushic et al., 2000; Song et al., 2008; Patras et al., 2013).
Estrous cycle management in mice by external hormone administration
has been widely used in experiments aimed at the study of vaginal
pathogen challenges in which cycle synchronization is required to
investigate susceptibility to selected pathogens (Kaushic et al.,
2000, 2003; Song et al., 2008; González et al., 2009; Patras et al.,
2013). This is the way in which murine vaginal infections caused by
Chlamydia trachomatis and Human Papilloma Virus were
maintained in animals in which a diestrous state had been induced by
treatment with exogenous progesterone (Kaushic et al., 2000; 2003). In
contrast, some vaginal opportunistic pathogens such as
Streptococcus agalactiae and Candida albicans or the
pathogen Neisseria gonorrhoeae, require a dominant estrogen
state to establish an infection in murine models (Song et al., 2008;
González et al., 2009; Patras et al., 2013). Although exogenous
treatment with progesterone or estrogen produces histological
similarities to diestrous or estrous states, the effects of these
hormones on murine estrous cycle, vaginal microbiota and immunity have
been rarely reported. Thus, knowledge of the effect of hormonal
treatments on these parameters is required. Moreover, it is useful
when standardization of animal protocols is needed. Therefore, the aim
of this work was to evaluate the effect of estradiol and progesterone
exogenous administration on estrous cycle, vaginal microbiota, and
immune cell populations of several organs in an experimental murine
model.
Materials and methods
Mice
Two-month-old female BALB/c mice weighing 25-30 g from the inbred
colony of CERELA (Centro de Referencia para Lactobacilos) were used.
The experiments were independently performed three times employing
three animals per experimental group and sampling day (Sd). Animals
were housed in plastic cages and fed ad libitum with a
conventional balanced diet, keeping their environmental conditions
constant. An intramuscular single dose of estradiol-hemisuccinate (EH)
(0.5 mg) (Eutocol, Craveri S.A.I.C. Laboratory, Buenos Aires,
Argentina) at day -2 or medroxyprogesterone-acetate (MPA) (2 mg)
(Medrosterona, Craveri S.A.I.C Laboratory, Buenos Aires, Argentina) at
day -5 was administered to mice to induce and maintain estrous (E) or
diestrous (DE) states, respectively (Silva de Ruiz et al., 2001;
Grangette et al., 2004; González et al., 2009; Li et al., 2010; Patras
et al., 2013). Both hormones were dissolved in saline. Day 0 was
considered the day on which the hormones began to exert their effect.
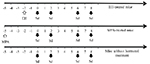
Thereafter, samples were taken on days 0, 2, 6 and 8 (Fig. 1).
Three animals per Sd, without hormonal injection, were included as
control groups. Samples were only taken from those
animals in E or DE states [determined from Papanicolaou (PAP)-stained
vaginal smears, as described below].
Experimental data obtained for each state on the different Sd were grouped in order to form control groups of mice in E (E-control) and DE (DE-control) states. The Institutional Laboratory Animal Care and Use Committee of CERELA approved the experimental protocol CRL-BIOT-LMP-2010/2A used in this work.
|
Figure 1. Schematic of administration of exogenous sex hormones and sampling days in BALB/c mice. The white up arrow indicates the day of hormone inoculation of mice with 0.5 mg estradiol-hemisuccinate (EH) or 2 mg medroxyprogesterone-acetate (MPA). The black down arrows indicate the days where the samples were taken and mice were sacrificed (Sd = sampling day). Click image to enlarge |
Sampling
Every Sd, vaginal washings (v.w.) were obtained under sterile conditions using automatic pipettes with tips loaded with 50 µl of 2% (w/v) foetal bovine serum (FBS, from NATOCOR, Cordoba, Argentina)-PBS (8.1 mM Na2HPO4, 1.5 mM KH2PO4, 140 mM NaCl, pH 7.2). Seven v.w. with fresh FBS–PBS were pooled from each mouse to be used for the different protocols. Subsequently, mice were anesthetized and subsequently bled by cardiac puncture and later dissected to remove vagina, femur (to isolate bone marrow cells) and spleen.
Determination of estrous cycle states
The induction and maintenance of the different states of the estrous cycle was evaluated and analyzed by cytological and histological techniques. Pictures were taken with an Axio Scope A1 Carl Zeiss microscope (Germany). The images were processed using Axio-Vision Release 4.8 software.
Cytological analysis
Aliquots of 20 µl v.w. were spread onto glass slides, immediately fixed in 96° alcohol, stained with PAP (Biopur, Santa Fe, Argentina) and observed by light microscopy at 400x. The different estrous cycle states (E and DE) were determined from the PAP-stained vaginal smears on the different Sd. One hundred cells were counted and classified as parabasal (BC) or intermediate cells (IC), basophilic (BSC) or eosinophilic (ESC) superficial cells, enucleated squama (ES) and cornified cell groups (G) (Silva de Ruiz et al., 2001). The results were expressed as the percentage of each type of cell in mice injected with hormones and in control groups.
Histological studies The vaginal tissues were fixed
in 4% (v/v) formaldehyde at 4°C and embedded in paraffin according to
standard histological methods. Sections were cut at 4 µm, stained with
hematoxylin-eosin (Biopur, Santa Fe, Argentina) and examined by light
microscopy at 400x. The different states of the estrous cycle were
determined according to OECD (2009).
Quantification of serum estradiol and progesterone
Serum was separated by centrifugation and frozen at -20°C. Quantification of estradiol and progesterone was carried out by the electro-chemiluminescence immunoassay (ECLIA) method with a COBAS 6000 CEE automatic analyzer (Roche Diagnostics, Mannheim, Germany).
Quantification of cultivable bacteria in vaginal washings
Appropriate dilutions of v.w. were plated on pH 6.4 De
Man-Rogosa-Sharpe (MRS) (Merck, Germany), MacConkey (Britania,
Argentina), Bile Esculin (Britania, Argentina), Mannitol Salt (MSA,
Britania, Argentina), pH 5.5 Lactobacillus Selective (LBS)
(Fluka, Switzerland), and pH 5.5 MRS agarized media to quantify
cultivable lactic acid bacteria, enterobacteria, enterococci,
staphylococci and lactobacilli, respectively. MacConkey, Bile Esculin
and MSA plates were incubated at 37°C for 24 h under aerobic
conditions, while MRS and LBS plates were incubated at 37°C for 48 to
72 h under anaerobic conditions. The number of microorganisms was
expressed as colony forming units (CFU)/ml of v.w.
Determination of total immune cells in v.w., bone marrow, spleen and
blood. The total number of immune cells in v.w., bone marrow (BM) and
spleen cell suspensions (described below) and blood was determined in
a Neubauer chamber by standard methodology. Furthermore, in blood
smears stained by the modified May Grünwald-Giemsa technique,
granulocyte and lymphocyte percentages were determined using an
optical microscope at 1000x magnification.
Evaluation of v.w., BM and spleen immune cells by flow cytometry
BM cells were isolated by flushing femurs with 2% FBS-PBS. Splenocytes were harvested by homogenization through a tissue strainer. Red blood cells were lysed by adding 2 ml of 10% lysing solution (Lysing Solution, BD Bioscience, Sparks, MD, USA). After that, v.w., BM and spleen cells were washed twice with 2% FBS-PBS by centrifugation at 500 g for 4 min at 4°C. In order to evaluate the expression of cell surface markers on leukocytes, v.w. (105 viable cells/tube), BM (106 viable cells/tube) and spleen (106 viable cells/tube) cell suspensions were pre-incubated with CD32/CD16 anti-mouse (Fc block) antibody for 15 min at 4°C. Then, cells were washed with 1% FBS-PBS and incubated with specific monoclonal antibodies conjugated with fluorochrome for 30 min at 4°C in the dark. The antibodies used for v.w. leukocytes and BM were fluorescein isothiocyanate (FITC)-labeled anti-mouse CD3, phycoerythrin (PE)-labeled anti-mouse Gr-1, and biotinylated anti-mouse B220. The antibodies for splenic cells were FITC-labeled anti-mouse CD3, PE-labeled anti-mouse CD24, and biotinylated anti-mouse B220. After incubation, cells were washed with 1% FBS-PBS. Samples treated with biotinylated antibodies were incubated with peridinin chlorophyll-α (PerCP)-labeled Streptovidin for 15 min at 4ºC. All the antibodies were obtained from BD Bioscience, San Diego, CA, USA. Then, cells were washed and re-suspended in 500 µl PBS. The samples were analyzed in a FACSCalibur cytometer (BD, CA, USA). Finally, the data were evaluated using the FlowJo software (7.6.5, TreeStar Inc., OR, USA). The leukocyte region was selected from forward scatter (FSC) versus side scatter (SSC) plots according to size (FSC) and cellular complexity (SSC). Afterwards, the expressions of the different markers under study were evaluated in the corresponding leukocyte gate. The CD3, Gr-1 and B220 markers allowed us to determine the behavior of T lymphocyte, myeloid cell and B lymphocyte populations, respectively. The B220/CD24 combination provided information about the degree of maturation of B cells.
Statistical analysis
Analysis of variance (ANOVA) using a general linear model was applied to determine statistically significant differences between values obtained on the different days post-inoculation of EH-treated and E-control mice, and between MPA-treated and DE-control mice. Moreover, the ANOVA-general linear model was also applied to determine the main and interaction effects of factors (type of hormone and sampling day). Significant differences between mean values were calculated with Tukey’s test using the MINITAB software (version 16 for Windows). A P value < 0.05 was considered as statistically significant.
Results
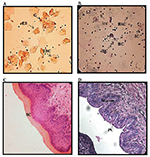
Effect of sex hormones on mice vaginal cytology and histology. EH administration to BABL/c mice induced the E state up to Sd 6 in 90% of the mice. On Sd 8, only 66% of the mice were in this state according to cytological and histological results. MPA administration induced the DE state up to Sd 2 in 90% of the mice. On Sd 6 and 8, 80% and 44% of the mice, respectively, were in the same state. Figure 2 summarizes the cytology and histological patterns obtained in mice treated with the sex hormones. The E state showed vaginal smears with ESC with a pyknotic core, ES and G (Fig. 2A). The histology of this state was characterized by the stratum corneum (Fig. 2C).
The DE state was apparent in vaginal smears with BSC, BC, IC and
leukocytes (Fig. 2B). In the histological smears, polygonal and plump
epithelial cells showing early mucification could be seen in the
superficial layers of the stratum germinativum (Fig. 2D).
|
Figure 2. Photomicrographs of PAP-stained
vaginal smears of A. estrous state from estradiol-hemisuccinate
(EH)-treated mice and B. DE state from
medroxyprogesterone-acetate (MPA)-treated mice. Vaginal slices
stained with Hematoxylin–Eosin C. estrous state from EH-treated
mice and D. DE state from MPA-treated mice (Magnification,
400x). BC: basal cells, IC: intermediate cells, BSC: basophilic
superficial cells, ESC: eosinophilic superficial cells, ES:
enucleated squama, G: cornified cell groups, leu: leukocyte, SC:
stratum corneum, SGerm: stratum geminativum and PC: polygonal
and plump cells. Click image to enlarge |
The cytological and histological patterns of the E and DE states
observed in mice treated with EH and MPA, respectively, were similar
to those of their respective controls (data not shown).
When analyzing the hormone effects on epithelial cell quantification,
no significant differences (P > 0.05) were found in the number of
each type of vaginal epithelial cells (ESC, BSC, IC, BC, ES, and G) on
the different Sd after the inoculation of each hormone. In the E state
of EH-treated mice, a significant predominance (P < 0.01) of ES
(53.55% ± 19) was observed with respect to all the cell types
quantified in this phase (ESC = 21% ± 11; G = 18% ± 15; BSC = 2.3% ±
4.6; IC = 3% ± 7 and BC = 2% ± 6). The ES number was significantly
higher with respect to all the other cells evaluated on Sd 0, 6 and 8
(Fig. 3A). In the DE state of MPA-treated mice, BC = 44.91% ± 7.6 were
higher (P = 0.03) than the other cells under study: ESC = 20% ± 6; BSC
= 17% ± 7.4; IC = 9.5% ± 8.5; ES = 8.3% ± 5.6 and G = 1.5% ± 2.5. The
number of BC was significantly higher than all the rest of the cells
quantified only on Sd 0, as indicated in Figure 3B.
When comparing the percentages for the various vaginal cells from mice
inoculated with hormones and their respective controls, no significant
differences (P > 0.05) were observed between both experimental
groups. These results, together with the qualitative observation of
the cytological and histological patterns of the animals, demonstrated
that the exogenous inoculation of hormones adequately induced the E
and DE states of the murine estrous cycle, without causing changes in
either vaginal cytology or histology.
|
Figure 3. Photomicrographs of PAP-stained
vaginal smears of A. estrous state from estradiol-hemisuccinate
(EH)-treated mice and B. DE state from
medroxyprogesterone-acetate (MPA)-treated mice. Vaginal slices
stained with Hematoxylin–Eosin C. estrous state from EH-treated
mice and D. DE state from MPA-treated mice (Magnification,
400x). BC: basal cells, IC: intermediate cells, BSC: basophilic
superficial cells, ESC: eosinophilic superficial cells, ES:
enucleated squama, G: cornified cell groups, leu: leukocyte, SC:
stratum corneum, SGerm: stratum geminativum and PC: polygonal
and plump cells. Click image to enlarge |
Quantification of hormone levels in serum
The hormone concentrations of EH-treated mice were 10.41 ± 1.19 pg
estradiol/ml serum and 1.79 ± 0.78 ng progesterone/ml. On Sd 0, the
14.4 pg estradiol/ml value obtained was significantly higher (P =
0.02) than in E-control mice (6.17 ± 1.16 pg estradiol/ml serum). On
the remaining Sd, no significant differences (P > 0.05) were
detected between these groups (Table 1).
MPA-treated mice showed hormone levels around 6.88 ± 2.71 pg
estradiol/ml and 0.21 ± 0.09 ng progesterone/ml. These levels were not
significantly different (P > 0.05) from those in DE-control mice
(Table 1).
Table 1. Levels of estradiol and progesterone in serum of mice intramuscularly administered with estradiol-hemisuccinate (EH) (0.5 mg) or medroxyprogesterone-acetate (MPA) (2 mg) and control animals.
| Experimental group | Sampling days |
Estradiol# (pg/ml serum)c |
Progesterone# (ng/ml serum)c |
| E-control mice | - | 6.17 ± 1.16b | 0.90 ± 0.01a |
| EH-treated mice | 0 | 14.40 ± 1.40a | 0.58 ± 0.51a |
| 2 | 6.10 ± 0.20ab | 0.30 ± 0.55a | |
| 6 | 9.65 ± 0.35ab | 0.15 ± 1.65a | |
| 8 | 8.98 ± 2.10ab | 1.03 ± 0.93a | |
| DE-control mice | - | 6.40 ± 1.40a | 2.14 ± 0.04a |
| MPA-treated mice | 0 | 5.00 ± 0.00a | 1.28 ± 0.19a |
| 2 | 9.30 ±1.20a | 1.15 ± 0.04a | |
| 6 | 8.30 ± 0.50a | 1.32 ± 0.04a | |
| 8 | 6.30 ± 0.79a | 1.33 ± 0.56a |
#Serum estradiol and progesterone were determined by the ECLIA method,
as described in the text. cData represent the mean value of pg
estradiol/ml or ng progesterone/ml ± standard error. Statistically
significant differences between the results obtained from EH or
MPA-treated mice on the different sampling days (Sd) with their
respective control are indicated by different letters (P <
0.05).
Sd were not included in control mice (without hormonal injection)
because the data from animals in estrous (E) or diestrous (DE) state
on the different Sd were grouped to form E-control or DE-control mice
groups, respectively.
Effect of sex hormones on the vaginal microbiota
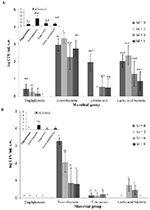
When evaluating the cultivable murine vaginal microbiota, the predominant microorganisms found were enterobacteria (2.42 ± 0.22 log CFU/ml) (P < 0.01) in all experimental groups. Moreover, lactic acid bacteria (1.01 ± 0.20 log CFU/ml) (including enterococci) were present in a significantly higher number (P = 0.04) compared with staphylococci (0.11 ± 0.05 log CFU/ml). Staphylococci were obtained only in low numbers (< 101 CFU/ml) in EH-treated and E-control mice. No cultivable lactobacilli grew in LBS and pH 5.5 MRS (Fig. 4).
In EH-treated animals, the numbers of enterobacteria and lactic acid
bacteria were not significantly different on all the days evaluated (P
> 0.05), while enterococci decreased on Sd 2 compared with day 0 (P
= 0.03) (Fig. 4A). In MPA-treated mice, enterobacteria decreased
significantly (P < 0.01) on Sd 6 and 8 with respect to day 0 (Fig.
4B), while the number of enterococci and lactic acid bacteria was not
significantly different (P > 0.05) for all Sd.
The comparison of the cultured vaginal microbiota of animals treated
with exogenous hormones and their control did not show statistically
significant differences between the different microbial groups
evaluated. Only, a significantly higher number (P = 0.02) of
enterobacteria was detected on Sd 0 in MPA-treated mice compared to
their control (Fig. 4).
Finally, higher numbers of cultivable microorganisms were obtained
from EH-treated mice compared to MPA-treated mice (P < 0.01) (Fig.
4). There was a significantly higher number of enterococci on Sd 0 of
EH-treated mice compared with MPA-treated mice (Fig. 4).
|
Figure 4. Effect of sex hormones on murine
vaginal microbiota. Viable counts of staphylococci,
enterobateria, enterococci, lactic acid bacteria from vaginal
washings (v.w.) of A. estradiol-hemisuccinate (EH)-treated mice
and estrous (E)-control mice and B. medroxyprogesterone-acetate
(MPA)-treated mice and diestrous (DE)-control mice. Data are
plotted as the mean number of viable cells (log CFU/ ml ±
standard error). Statistically significant differences between
the results obtained from EH or MPA-treated mice at the
different sampling days (Sd) with their respective control are
indicated by different letters (P < 0.05). Differences
between EH-treated and MPA-treated mice at the different Sd are
indicated by * (P < 0.05). Click image to enlarge |
Effect of sex hormones on immune cells
Immune cell quantification in v.w., BM, spleen and blood. When evaluating the number of leukocytes in v.w., a significant higher number (P = 0.02) in EH-treated mice was found on Sd 8 (8 x 105 cells/ml of v.w.) compared with the other days under evaluation and with E-control mice. In the MPA-treated mice, leukocyte numbers showed no statistically significant differences (P > 0.05) on all the days evaluated (around 1.3 to 2.8 x 105 cells/ml of v.w.). However, when comparing MPA-treated mice with DE-control animals, MPA administration significantly decreased (P = 0.04) the leukocyte numbers on Sd 0, 2 and 6. Moreover, no significant differences were observed between leukocyte numbers in v.w. of EH-treated mice and those of MPA-treated mice on all the days assayed (Table 2).
Table 2. Total number of leukocytes from vaginal washings of mice intramuscularly administered with estradiol-hemisuccinate (EH) (0.5 mg) or medroxyprogesterone-acetate (2 mg) (MPA) and control animals.
| Experimental group | Sampling days |
Total leukocytes# (x 105)/ml of v.w. |
| E-control mice | - | 1.11 ± 0.56b |
| EH-treated mice | 0 | 0.22 ± 0.03b |
| 2 | 1.96 ± 1.28b | |
| 6 | 0.91 ± 0.22b | |
| 8 | 8.02 ± 2.71a | |
| DE-control mice | - | 13.79 ± 3.60a |
| MPA-treated mice | 0 | 2.77 ± 0.42b |
| 2 | 1.40 ± 0.38b | |
| 6 | 1.38 ± 0.54b |
#Data represent the mean number of leukocytes ± standard error from
vaginal washing (v.w.). Statistically significant differences between
the results obtained from EH or MPA-treated mice at the different
sampling days (Sd) with their respective control are indicated by
different letters (P < 0.05).
Sd were not included in control mice (without hormonal injection)
because the data obtained from animals in estrous (E) or diestrous
(DE) state on the different Sd were grouped to form E-control or
DE-control mice groups, respectively.
Neither EH nor MPA administration induced significant modifications (P > 0.05) in the total immune cell number of BM, spleen or peripheral blood on all the days evaluated. Moreover, the number of leukocytes in these organs and in blood were not significantly different (P > 0.05) from those obtained from control animals and between hormonal treatments, and were around 3 x 107 cells/femur, 4 x 107 cells/spleen and 7 x 109 cells/ml in blood. Differential quantification of leukocytes in blood was not affected by hormonal treatments when compared to control animals (data not shown).
Phenotyping of v.w., BM and spleen immune cells
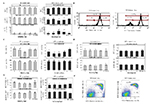
When evaluating the effect of sex hormones on myeloid and lymphoid immune cell populations of v.w., only Gr-1+ cells were observed. No CD3+ or B220+ cells were detected in v.w. EH did not modify the percentage of Gr-1+ cells with respect to Sd or E-control animals. However, MPA significantly reduced (P = 0.02) the Gr-1+ cell percentage on Sd 0 with respect to the other days and to DE-control animals (Fig. 5A).
On the other hand, two different expression profiles (high and low) of
Gr-1 marker were detected in the Gr-1+ cell population (Fig. 5A, 5B).
Gr-1high cells in v.w. could represent neutrophils, while Gr-1low
cells could correspond to other granulocyte populations and
macrophages (Herrera et al., 2014). The percentage of Gr-1high and
Gr-1low cells obtained on all Sd following the two hormonal treatments
was not significantly different (P > 0.05) compared to their
respective controls. When evaluating the expression of this marker
between the EH and MPA treatments, a significantly higher percentage
(P < 0.01) of Gr-1high cells (92.34% ± 2.09) and a lower percentage
of Gr-1low cells (2.10% ± 1.31) (P < 0.01) were obtained in
EH-treated mice than in MPA-treated mice (74.60% ± 5.62 and 20.70% ±
5.92 of Gr-1high and Gr-1low cells respectively) on all Sd (Fig. 5A,
5B).
When determining the effect of hormonal treatments on the lymphoid
cell population of BM, a lower percentage of CD3 cells (1.91% ± 0.11)
compared to B220 cells (88.13% ± 0.81) was observed in the
cytometry-lymphocyte region in all experimental groups (Fig. 5C). The
percentage of CD3+ cells in EH-treated mice was not statistically
different (P > 0.05) from the E-control animals on all the Sd
evaluated. However, in MPA-treated mice a lower percentage (P = 0.041)
of CD3+ cells on Sd 2 and 6 with respect to DE-control mice was
obtained. When comparing EH and MPA treatments, a significantly higher
expression (P < 0.01) of CD3+ cells was observed in EH-treated mice
than in MPA-treated mice on Sd 2 (Fig. 5C). When the B220 marker
expression was evaluated in EH-treated mice, a significantly lower
cell percentage (P = 0.03) was obtained on Sd 6 compared with the
other days under study and with E-control animals. No differences were
found in the percentage of B220+ cells in MPA-treated mice compared
with their respective control or between the two hormonal treatments
(Fig. 5C). The evaluation of Gr-1+ cells in the cytometry-granulocytes
region showed no statistically significant differences (P > 0.05)
among the Sd and control animals, or between hormonal treatments (data
not shown).
In spleen, the percentage of CD3+ lymphocytes was higher (50.09 ±
1.38) in all the experimental groups compared with B220+ lymphocytes
(41.03 ± 1.21). The total populations of CD3+ and B220+ cells in
spleen were not statistically significant (P > 0.05) between all Sd
and control animals, and between hormonal treatments, as shown in
Figure 5D. On the other hand, as spleen is the organ where B
lymphocyte maturation is completed, the expression of B220 vs CD24 was
also evaluated in the cytometry-lymphocyte region. Two different
expression profiles of the B220highCD24low cells (B-mature
lymphocytes) and B220lowCD24high cells (immature B lymphocytes) were
studied (Barbieri et al., 2013). When evaluating the effect of
hormonal administration on B-lymphocyte maturation, neither treatment
modified the percentage of B220highCD24low or B220lowCD24high cell
populations with respect to their controls (Fig. 5E). When EH and MPA
treatments were compared, a significantly lower percentage of immature
B lymphocytes and a higher percentage of mature B lymphocytes in
MPA-treated mice compared to EH-treated mice were observed (P <
0.01). This difference was significant only on Sd 0 (Fig. 5E, 5F).
|
Figure 5. Effect of sex hormones on cells of
murine immune system. Percentage of A. Gr-1+, Gr-1high and
Gr-1low cells from vaginal washing (v.w.), C. CD3+ and B220+
cells from bone marrow (BM), D. CD3+ and B220+ cells from
spleen, and E. B220highCD24low and B220lowCD24high cells from
spleen of estradiol-hemisuccinate (EH)-treated, estrous
(E)-control, medroxyprogesterone-acetate (MPA)-treated and
diestrous (DE)-control mice. Data are plotted as the mean values
of percentage of cells ± standard error. Statistically
significant differences between the results obtained from EH or
MPA-treated mice at the different sampling days with their
respective control are indicated by different letters (P <
0.05). Differences between EH-treated and MPA-treated mice at
the different sampling days are indicated by *(P < 0.05). B.
Histograms of EH and MPA-treated mice, obtained by flow
cytometry, in which the high and low expression of Gr-1 marker
was observed. F. Representative dot plot of B220 vs CD24
expression obtained by flow cytometry in EH and MPA-treated
mice. Click image to enlarge |
Discussion
In several murine models of vaginal infection, some investigators have demonstrated that inoculation using different routes and doses (0.01 to 0.5 mg) of estradiol derivatives generated an estrous state at 2 days post-inoculation. This state persisted for a week and allowed an effective colonization of various microorganisms (González et al., 2009; Pietrella et al., 2011). On the other hand, other studies reported that 2 to 2.5 mg doses of different progesterone derivatives induced a diestrous state at day 5 after hormonal inoculation (Grangette et al., 2004; Li et al., 2010). On the basis of these studies, the effect of 0.5 mg of EH and 2 mg of MPA was evaluated in the present investigation and similar results were obtained. The cytological and histological evaluations performed showed that EH administration induced an E state after 2 days of hormonal inoculation, while MPA administration induced a DE state at day 5 post-inoculation. Both states were maintained in most of the animals for approximately 6 days. Following these determinations, the effect of sex hormones was evaluated in different murine organs.
As in human beings, the murine reproductive tract undergoes structural
changes during the estrous cycle. The human follicular proliferative
phase is comparable to proestrous and estrous states of the murine
estrous cycle, in which estrogens predominate, whereas the human
secretory phase is analogous to mouse metaestrous and diestrous
stages, in which progesterone predominates (Hickey et al., 2012). When
evaluating the estradiol and progesterone serum levels in mice treated
with exogenous sex hormones, slight differences were noted with
respect to the hormonal levels of their controls, suggesting that the
protocols applied induced similar states to the physiological
modifications of the murine estrous cycle. Serum estradiol and
progesterone levels of EH-treated mice were within the physiological
range reported by Sasaki et al. (2009) and Byers et al. (2012) in
mice. Only on Sd 0 were serum estradiol levels higher than those of
E-control animals. In a similar way, Medina & Kincade (1994)
reported an increase in serum estrogen levels shortly after the
inoculation of 1 mg of estradiol in BALB/c mice, which were normalized
at day 3. On the other hand, estradiol levels of MPA-treated mice
presented values similar to those reported for a diestrous state
(Sasaki et al., 2009; Byers et al., 2012). However, the progesterone
levels detected in these animals were lower than the ones reported in
diestrous, but close to those published by Sander et al. (2009) in
BALB/c mice, where regression of the corpus luteum was observed, a
state in which serum progesterone levels declined (a phase
corresponding to late diestrus).
Sex hormones produce numerous physiological modifications in the
vaginal tract, including changes in carbohydrate and protein
metabolism that could alter the host’s indigenous microbiota and
the characteristics of the tissues colonized by microorganisms
(Eschenbach et al., 2000). Analytical methods of cultivation and
isolation have been widely applied in several laboratory animal
species in order to evaluate their vaginal microbiota (Meysick &
Garber, 1992; Noguchi et al., 2003). Thus, in the present work one of
the objectives was to evaluate whether the administration of exogenous
hormones induced some modification in the cultivable vaginal
microbiota compared to control animals. The results obtained showed
that neither of the two hormonal treatments altered the cultivable
indigenous vaginal microbiota compared to their respective control.
Moreover, a higher number of aerobic microorganisms was obtained in
EH-treated animals compared to those treated with MPA. The predominant
cultivable microorganisms in the murine vaginal tract of the BALB/c
mice were enterobacteria, followed by lactic acid bacteria (among them
enterococci) and lastly staphylococci (only detected in very low
numbers in EH-treated and E-control mice). Cultured lactobacilli were
not isolated from hormone-treated or control animals. In a similar
way, other researchers have applied the cultured-dependent technique
to evaluate some of the vaginal microbiota modifications: Noguchi et
al. (2003) demonstrated by culture techniques a higher number of
bacteria during estrous than diestrous and low to absent numbers of
anaerobes in vagina of female ICR/Kud mice (age, 12 weeks). Different
to our results, these authors were able to detect lactobacilli in LBS
agar. Also, Meysick & Garber (1992) demonstrated by standard
microbiological techniques that the vaginal microbiota of BALB/c mice
(22-24 g) consisted mainly of S. aureus and
Enterococcus species (32-76%), followed by lactobacilli and
enteric bacilli (16-32%), and a low prevalence of both anaerobic
species and coagulase negative staphylococci (4-16%). Moreover, these
authors, as in our results, have shown that the estrogenization of
mice with 0.5 mg of estradiol valerate did not significantly modify
the vaginal microbiota, but there was a slight increase in the number
of bacterial species isolated per mouse. In contrast to our results,
Voronkova et al. (2008) reported that lactobacilli were the dominant
bacteria in the mouse vaginal tract. Moreover, they found different
genera of aerobic bacteria, including
Streptococcus, Staphylococcus, Micrococcus, Bacillus and some
anaerobic bacteria such as
Fusobacterium, Peptococcus, Peptostreptococcus and
Bacteroides. Taking into account that the cultured-based
technique, frequently applied in our laboratory and used in the
present work does not allow the isolation of all microorganisms,
further studies should be carried out by applying molecular
methodologies to evaluate the effect of administration of exogenous
hormones on murine vaginal microbial populations.
Experimental animal studies have shown that estrogen and progesterone
modulate immune reactivity by modifying the number of granulocytes,
inflammation mediated by T cells, cytotoxicity mediated by natural
killer cells and production of immunoglobulins (Josefsson et al.,
1992; Nilsson & Carlsten, 1994; Attanasio et al., 2002). Based on
these findings, the sex hormone effect on the immune cells of vagina,
BM, spleen and blood was evaluated to determine if these two hormonal
treatments altered the murine immune system. Evaluation of the effect
of these sex hormones on the immune cells present in v.w. revealed
that the EH treatment did not modify the number of total leukocytes
with respect to control animals, except on Sd 8. On that day, higher
numbers of leukocytes were detected in EH-treated mice compared to
E-control mice and the remaining Sd. These results could be explained
by the gradual loss of the hormone over the course of the study and by
the fact that mice could pass from estrous to metaestrous, a period
characterized by a large number of leukocytes in v.w. (Mclean et al.,
2012). When the MPA treatment was applied, a decrease in total
leukocyte numbers was observed compared to control animals. In
addition, no differences were found in the total numbers of leukocytes
in v.w. of EH- and MPA-treated mice. These results indicate that MPA
treatment modified vaginal leukocytes since the diestrous state of the
estrous cycle is characterized by a higher leukocyte number than the
estrous state (Mclean et al., 2012). Considering the cytological and
histological similarities between MPA-treated and DE control mice and
the hormonal levels discussed above, we can suggest that MPA treatment
induced a state similar to late DE.
Flow cytometry studies showed that neutrophils were the predominant
cells in the leukocyte population of murine v.w. Populations of T and
B lymphocytes were not detected. Giraldo et al. (2012) evaluated
immune cells present in v.w. of 142 women with a diagnosis of
bacterial vaginosis, vulvovaginal candidiasis or normal microbiota by
flow cytometry and reported that neutrophils were the predominant
leukocytes in all the samples and that macrophages and T and B
lymphocytes were either absent or present at very low percentages.
Evaluation of the effect of sex hormones on the granulocyte population
of v.w. by flow cytometry showed that treatment with EH did not modify
the percentage of Gr-1+ cells in v.w. compared to control animals,
whereas inoculation of MPA decreased the percentage of Gr-1+ cells
only on Sd 0. In addition, no significant differences between the two
treatments were observed in the percentage of Gr-1+ cells. However,
differences in the expression of Gr-1+ cells in v.w. between hormonal
treatments were obtained. A higher percentage of Gr-1high cells
(characteristic of neutrophils) and a lower percentage of Gr-1low
cells (macrophages or other granulocytes) were observed in EH-treated
mice compared to those treated with MPA. The high and low patterns in
the Gr-1+ population in the two hormonal treatments were not
significantly different from their respective controls. These results
suggest that during the E states of estrous cycle in which estrogens
predominate, neutrophils are the prevailing leukocytes. However, in
the DE state where progesterone is dominant, other cell types such as
monocytes and macrophages could be present. On this subject, there is
evidence that some cells of the immune response are regulated in the
middle of the menstrual cycle by estrogens in order to prevent an
immunological attack on the fetus during pregnancy (Lee & Chiang,
2012).
The study of the effect of sex hormones on cells involved in the
systemic immune response showed that neither the total number of
leukocytes in BM and spleen nor the differential and total number of
leukocytes in peripheral blood were modified by the hormonal
treatments. Similarly, Tikare et al. (2008) reported that there were
no significant differences in the total number and differential
characterization of leukocytes in blood during the different phases of
the menstrual cycle in women. However, Smith et al. (2007) detected an
increased number of neutrophils in human blood when estrogen levels
were higher during the menstrual cycle.
The two hormonal treatments did not produce significant differences in
the myeloid populations of BM, but caused a slight decrease in T and B
lymphoid populations. EH administration reduced B220+ cells only on Sd
6, while MPA affected CD3+ cells on Sd 2 and 6. Taking into account
that several studies performed in animal experimental models have
reported the suppressive effect of estrogen on T and B lymphopoiesis,
and that neither estrogen nor progesterone modified the number or role
of myeloid and granulocytes cells in BM (Medina et al., 1993; Medina
& Kincade, 1994; Medina et al., 2000; Bhavanam et al., 2008), it
is possible to suggest that the hormonal treatments applied in the
present investigation slightly affected the lymphoid population of BM
without affecting animal welfare.
Cytometric studies performed by De León-Nava et al. (2009) in BALB/c
female mice demonstrated that gonadectomy did not affect the
percentage of T and B populations in spleen. Moreover, Medina et al.
(1993) and Medina & Kincade (1994) reported that mature B
lymphocyte populations in the spleen of mice were not significantly
modified during pregnancy or after estrogen treatment. These reports
suggest that sex hormones do not induce important modifications in
splenic immune cells. In a similar way, the evaluation of immune cells
in spleen demonstrated that neither of the two hormonal treatments
affected CD3+ and B220+ cell populations or mature and immature B
lymphocyte populations compared to their control. However, when the
degree of B-cell maturation in the spleen was compared between the two
hormonal treatments, a higher number of mature B cells
(B220highCD24low) was observed in MPA-treated mice compared to
EH-treated mice. It is noteworthy that Medina et al. (1993) and Medina
& Kincade (1994) reported a partial depletion of a subset of B
cells characterized by a high expression of CD24 in pregnant mice
compared to non-pregnant mice. Taking into account these reports and
our results, it is possible to suggest that higher progesterone levels
could induce B lymphocyte maturation.
In conclusion, our results demonstrate that exogenous inoculation of
estrogen and progesterone, despite slight changes induced in some of
the parameters evaluated compared to untreated animals, allowed the
establishment of adequately standardized and consistent experimental
protocols for further studies. Moreover, this work shows the
complexity of the endocrine interactions with different constituents
of the vaginal tract and at the systemic level. Inoculation of
estrogens allows the isolation of a higher number of autochthonous
microorganisms in mice vagina, while progesterone promotes the
presence of myeloid cells participating in the innate immune response
of vagina and the maturation of B lymphocytes in spleen.
Acknowledgements
This paper was supported by CONICET (Consejo Nacional de
Investigaciones Científicas y Técnicas, Argentina) (PIP 545) and
ANPCyT (Agencia Nacional de Promoción Científica y Tecnológica) (PICT
2013-1187). We thank Elena Bru for her help with the statistical
analysis of experimental results.
References
- Attanasio R, D Gust, M Wilson, T Meeker & T Gordon: Immunomodulatory effects of estrogen and progesterone replacement in a nonhuman primate model. J. Clin. Immunol., 2002, 22, 263–269. https://www.ncbi.nlm.nih.gov/pubmed/12405159
- Barbieri N, J Villena, MHerrera, S Salva & S Alvarez: Nasally administered Lactobacillus rhamnosus accelerate the recovery of humoral immunity in B lymphocyte-deficient malnourished mice. J. Nutr., 2013, 143, 227-235. doi: 10.3945/jn.112.165811
- Bhavanam S, DP Snider & C Kaushic: Intranasal and subcutaneous immunization under the effect of estradiol leads to better protection against genital HSV-2 challenge compared to progesterone. Vaccine, 2008, 26, 6165–6172. doi: 10.1016/j.vaccine.2008.08.045
- Byers SL, MV Wiles, SL Dunn & RA Taft: Mouse Estrous Cycle Identification Tool and Images. PLoS One, 2012, 7, e35538. doi: 10.1371/journal.pone.0035538
- De León-nava MA, K Nava, G Soldevila, L López-Griego, JR Chávez-Ríos, JA Vargas-Villavicencio & J Morales-Montor: Immune sexual dimorphism: Effect of gonadal steroids on the expression of cytokines, sex steroid receptors, and lymphocyte proliferation. J. Steroid. Biochem. Mol. Biol., 2009, 113, 57–64. doi: 10.1016/j.jsbmb.2008.11.003
- Eschenbach DA, SS Thwin, DL Patton, TM Hooton, AE Stapleton, K Agnew, C Winter, A Meier & WE Stamm: Influence of the normal menstrual cycle on vaginal tissue, discharge, and microflora. Clin. Infect. Dis., 2000, 20, 901–907. doi: 10.1086/313818
- Giraldo P, J de Carvalho, RL do Amaral, AK da Silveira Gonçalves, JJr Eleutério & F Guimarães: Identification of immune cells by flow cytometry in vaginal lavages from women with vulvovaginitis and normal microflora. Am. J. Reprod. Immunol., 2012, 67, 198–205. doi:10.1111/j.1600-0897.2011.01093.x
- González GM, E Robledo, E Garza-González, M Elizondo & JG González: Efficacy of albaconazole against Candida albicans in a vaginitis model. Antimicrob. Agent. Chemother., 2009, 53, 4540–4541. doi: 10.1128/AAC.00565-09
- Grangette C, H Müller-Alouf, P Hols, D Goudercourt, J Delcour, M Turneer & A Mercenier: Enhanced mucosal delivery of antigen with cell wall mutants of lactic acid bacteria. Infect. Immun., 2004, 72, 2731–2737. doi: 10.1128/IAI.72.5.2731-2737.2004
- Herrera M, S Salva, J Villena, N Barbieri, G Marranzino & S Alvarez: Dietary supplementation with lactobacilli improves emergency granulopoiesis in protein-malnourished mice and enhances respiratory innate immune response. PLoS One, 2014, 9, e90227. doi: 10.1371/journal.pone.0090227
- Hickey DK, JV Fahey & CR Wira: Mouse estrous cycle regulation of vaginal versus uterine cytokines, chemokines, α-/β-defensins and TLRs. Innate. Immun., 2012, 19, 121-131. doi: 10.1177/1753425912454026
- Josefsson E, A Tarkowski & H Carlsten: Anti-inflammatory properties of estrogen. I. In vivo suppression of leukocyte production in bone marrow and redistribution of peripheral blood neutrophils. Cell. Immunol., 1992, 142, 67–78. doi: 10.1016/0008-8749(92)90269-U
- Kaushic C, AA Ashkar, LA Reid & KL Rosenthal: Progesterone increases susceptibility and decreases immune responses to genital herpes infection. Am. Soc. Microbiol., 2003, 77, 4558–4565. doi: 10.1128/JVI.77.8.4558-4565.2003
- Kaushic C, FAN Zhou, AD Murdin & CR Wira: Effects of estradiol and progesterone on susceptibility and early immune responses to Chlamydia trachomatis infection in the female reproductive tract. Infect. Immun., 2000, 68, 4207–4216. doi: 10.1128/IAI.68.7.4207-4216.2000
- Larsen B: Vaginal flora in health and disease. Clin. Obstet. Gynecol., 1993, 36, 107–121. https://www.ncbi.nlm.nih.gov/pubmed/8435935
- Lee T & B Chiang: Sex differences in spontaneous versus induced animal models of autoimmunity. Autoimmun. Rev., 2012, 11, A422–A429. doi: 10.1016/j.autrev.2011.11.020
- Li L, Y Yang, SH Yuan, YM Wan, C Qiu, YL Feng, JQ Xu & XY Zhang: Establishing a Th17 based mouse model for preclinical assessment of the toxicity of candidate microbicides. Chin. Med. J., 2010, 123, 3381–3388. doi: 10.3760/cma.j.issn.0366-6999.2010.23.002
- Mclean AC, N Valenzuela, S Fai & SA Bennett: Performing vaginal lavage, crystal violet staining, and vaginal cytological evaluation for mouse estrous cycle staging identification. J. Vis. Exp., 2012, 67, e4389. doi: 10.3791/4389
- Medina KL, G Smithson & PW Kincade: Suppression of B lymphopoeisis during nomial pregnancy. J. Exp. Med., 1993, 178, 1507-1515. doi: 10.1084/jem.178.5.1507
- Medina KL & PW Kincade: Pregnancy-related steroids are potential negative regulators of B lymphopoiesis. Proc. Natl. Acad. Sci. USA., 1994, 91, 5382–5386. doi: 10.1073/pnas.91.12.5382
-
Medina KL, A Strasser & PW Kincade: Estrogen influences the
differentiation, proliferation, and survival of early B-lineage
precursors. Immunology, 2000, 95, 2059–2068.
http://www.bloodjournal.org/content/95/6/2059.long
Meysick K & Garber G: Interactions between Trichomonas vaginalis and vaginal flora in a mouse model. J. Parasitol., 1992, 78, 157–160. doi: 10.2307/3283708 - Nilsson N & H Carlsten: Estrogen induces suppression of natural killer cell cytotoxicity and augmentation of polyclonal B cell activation. Cell. Immunol., 1994, 158, 131–139. doi: 10.1006/cimm.1994.1262
- Noguchi K, K Tsukumi & T Urano: Qualitative and quantitative differences in normal vaginal flora of conventionally reared mice, rats, hamsters, rabbits, and dogs. Comp. Med., 2003, 404-412.
- OECD: Guidance document for histologic evaluation of endocrine and reproductive tests in rodents. OECD Series on testing and assessment N° 106. Organ. Econ. Coop. Dev. Paris. 2009, 26 pp. https://www.oecd.org/chemicalsafety/testing/43754831.pdf
- Patras KA, NY Wang, EM Fletcher, CK Cavaco, A Jimenez, M Garg, J Fierer, TR Sheen, L Rajagopal, KS Doran: Group B Streptococcus CovR regulation modulates host immune signalling pathways to promote vaginal colonization. Cell. Microbiol., 2013, 15, 1154–1167. doi: 10.1111/cmi.12105
-
Pietrella D, A Rachini, M Pines, N Pandey, P Mosci, F Bistoni, C
d’Enfert & A Vecchiarelli: Th17 Cells and IL-17 in
protective immunity to vaginal candidiasis. PLoS One, 2011, 6,
e22770. doi: 10.1371/journal.pone.0022770
Sakiani S, NJ Olsen & WJ Kovacs: Gonadal steroids and humoral immunity. Nat. Rev. Endocrinol., 2013, 9, 56–62. doi: 10.1038/nrendo.2012.206 - Sander VA, GB Facorro, L Piehl, E Rubín de Celis & AB Motta: Effect of DHEA and metformin on corpus luteum in mice. Reproduction, 2009, 138, 571–579. doi: 10.1530/REP-08-0325
- Sasaki S, K Nagata & Y Kobayashi: Regulation of the estrous cycle by neutrophil infiltration into the vagina. Biochem. Biophys. Res. Commun., 2009, 382, 35–40. doi: 10.1016/j.bbrc.2009.02.112
- Silva de Ruiz C, MR Rey, A Pesce de Ruiz Holgado & ME Nader-Macías: Experimental administration of estradiol on the colonization of Lactobacillus fermentum and Escherichia coli in the urogenital tract of mice. Biol. Pharm. Bull., 2001, 24, 127–134. doi: 10.1248/bpb.24.127
-
Smith JM, Z Shen, CR Wira, MW Fanger & L Shen: Effects of
menstrual cycle status and gender on human neutrophil phenotype. Am.
J. Reprod. Immunol., 2007, 111–119. doi:
10.1111/j.1600-0897.2007.00494.x
Song W, S Condron, BT Mocca, SJ Veit, D Hill, A Abbas & AE Jerse: Local and humoral immune responses against primary and repeat Neisseria gonorrhoeae genital tract infections of 17β-estradiol-treated mice. Vaccine, 2008, 26, 5741–5751. doi: 10.1016/j.vaccine.2008.08.020 - Tikare SN, KK Das & SA Dhundasi: Blood leukocyte profile in different phases of menstrual cycle. Indian. J. Physiol. Pharmacol., 2008, 54, 201–204. https://www.ncbi.nlm.nih.gov/pubmed/19130867
- Voronkova OS, EA Sirokvasha & AI Vinnikov: Experimental vaginal dysbiosis on the model of white laboratory mice. Mikrobiol. Z., 2008, 70, 47–58. https://www.ncbi.nlm.nih.gov/pubmed/19351049
- Wira CR, JV Fahey, M Ghosh, MV Patel, DK Hickey & DO Ochiel: Sex hormone regulation of innate immunity in the female reproductive tract: The role of epithelial cells in balancing reproductive potential with protection against sexually transmitted pathogens. Am. J. Reprod. Immunol., 2010, 63, 544–565. doi: 10.1111/j.1600-0897.2010.00842.x