Technical Report
Rederivation of transgenic rodent models expressing disease modified tau protein - a report
by Bernadeta Valachova2, Veronika Cubinkova1,2, Ivana Uhrinova1,2, Veronika Brezovakova1, Jozef Hanes1,2, Santosh Jadhav1,2*
1Institute of Neuroimmunology, Slovak Academy of Sciences, Centre of
Excellence for Alzheimer’s Disease and Related Disorders,
Dubravska 9, 845 10 Bratislava, Slovak Republic
2Axon Neuroscience R&D Services SE, Dvořákovo nábrežie 10,
Bratislava, Slovak Republic
 Correspondence: Santosh Jadhav
Correspondence: Santosh Jadhav
Institute of Neuroimmunology, Slovak Academy of Sciences
Dubravska Cesta 9, 845 10 Bratislava, Slovak Republic
santosh.jadhav@savba.sk
fax: 00412 2 5477 4276
tel: 00412 2 5478 8100
Summary
Transgenic animals are used extensively as in vivo experimental systems for modelling human diseases. A prerequisite for obtaining reliable experimental results is that the animal models used are free of defined infectious agents, including those that rarely produce disease, but still can modulate immune responses and alter the phenotype of affected animals. We have recently rederived transgenic rodent models of tauopathy to meet specific-pathogen free health standards for breeding in barrier areas. The surgical implantation of 2- cell embryos in three transgenic rat lines SHR24, SHR72 and W72 resulted in a total of 86 pups, out of which 36 were positive for transgene (42% efficiency). An overall 48 % transgenic efficiency was achieved in two transgenic mice lines R3m/4 and R3m/7 following non-surgical transfer of embryos. Microbiological evaluation, based on sentinel screening and serological examinations, confirmed the SPF status of all rederived strains. Most importantly, all rederived transgenic rodents developed AT8 positive neurofibrillary structures demonstrating high reproducibility of the phenotype. To the best of our knowledge, this report is the first on rederivation of transgenic models of human tauopathy.
Keywords Rederivation; non-surgical embryo transfer; surgical implantation; transgenic model
Introduction
ICurrently, the vast majority of biomedical studies are performed on specific pathogen free (SPF) animals which need to be free of pathogens that may affect animal welfare and confound the validity of experimental data (Van Keuren and Saunders, 2004; Shek et al., 2008; Amstislavsky et al., 2013; Mähler et al., 2014). Rederivation enables the production of SPF animals from potentially infected rodents. It can be achieved either by hysterectomy of late-term fetuses, cross-fostering of new born pups to clean surrogate mothers or by transfer of preimplantation embryos to recipient females (Artwohl et al., 2008). The technique preferred depends on several factors and is highly influenced by the health condition of animals, colony size, number of staff involved and their personal skills, as well as financial considerations and barrier facility design (Fray et al., 2008). Although both cross-fostering and hysterectomy are useful to rescue poorly breeding strains, they are not efficient enough against fetoplacental infection caused by pathogens, such as Listeria monocytogenes, Murine norovirus (MNV) and Helicobacter hepaticus (Le Monnier et al., 2006; Yeom et al., 2009). In contrast, rederivation by embryo transfer (ET) provides full protection against pathogens, and thus represents the most secure way of introducing animals into barrier colonies (Suzuki et al., 1996; Morrell, 1999; Van Keuren and Saunders, 2004; Du et al., 2010; Sztein et al., 2011; Raspa et al., 2016). Surgical transfer of rodent embryos, with respect to their developmental stage, can be carried out either by the puncture of the oviductal wall/ uterine horn or through the uterotubal junction (Mclaren and Michie, 1956; Sato et al., 1999; Chin et al., 2001; Sarvari et al., 2013; Bermejo-Alvarez et al., 2014). A non-surgical method using a non-surgical embryo transfer (NSET) device (ParaTechs, Lexington, KY) has recently been developed as a refinement to surgical uterine transfer in mice. This approach is technically based on the insertion of a catheter containing blastocysts through the cervical canal into the lumen of the uterine horn (Green et al., 2009). Recently, we have established a rederivation protocol by means of embryo transfer in order to generate SPF transgenic animals. Here we report the rederivation of transgenic rodent models of human tauopathy and evaluate the neuropathological phenotype of the transgene positive animals.
Materials & Methods
Transgenic animals
The following transgenic models of tauopathy expressing human
truncated tau aa151-391 have been rederived: models with 3
microtubule-binding repeats (3R), i.e. SHR24 rat (Filipcik
et al., 2012) as well as R3m/7 and R3m/4 mice lines (Zimova
et al., 2016), and models with 4 microtubule-binding repeats
(4R) i.e. SHR72 (Zilka et al., 2006) and W72 rats (Stozicka
et al., 2010). These animals were used as donors of the
embryos. The generation and characterization of most of these
transgenic lines have been previously described (Zilka
et al., 2006; Koson et al., 2008; Stozicka
et al., 2010; Filipcik et al., 2012; Zimova
et al., 2016). The characterization of R3m/7 is currently
underway. All these transgenic lines are hemizygous for the transgene
and express human truncated tau under Thy-1 promoter. Briefly, the
transgene construct was prepared by ligation of a cDNA coding for
human tau protein truncated at amino acid positions 151-391 into the
mouse Thy-1 gene downstream of the brain promoter/enhancer sequence.
The part of original Thy-1 gene sequence coding for exons II-IV
together with the thymus enhancer sequence was replaced by the cDNA.
The transgenic DNA was linearized by cleavage with EcoRI and all
vector sequences were removed prior to microinjection. Transgenic rats
were generated by pronuclear microinjection into 1-day old SHR embryos
and similarly, the transgenic mice by microinjection into 1-day old
C57BL/6NCrl embryos. Founders were double screened by polymerase chain
reaction (PCR) using Thy-1-specific and human tau specific primers
amplifying START codon (forward: 5´-GTGGATCTCAAGCCCTCAAG-3´, reverse:
5´-CCTGATTTTGGAGGT-3´) and STOP codon (forward: 5´-
CCTGATTTTGGAGGT-3´, reverse: 5´-TATGCATGGAGGGAGAAG-3´) flanking
sequences (Zilka et al., 2006). Phenotypically, the rodents
develop cardinal features of tauopathies, such as progressive age
dependent tau hyperphosphorylation, formation of neurofibrillary
tangles and ultimately reduced lifespan (Zilka et al., 2006;
Filipcik et al., 2012; Zimova et al., 2016).
Recipient animals
For surgical implantation of transgenic rat embryos, Crl: CD (SD)
(Velaz, Prague, Czech Republic) rats in the SPF state were used as
recipients.
Outbred Crl: CD1 (ICR) (CD-1) mice were first purchased from Charles
River Laboratories (Wilmington, MA, USA) and then bred under strictly
regulated conditions of the barrier facility. These mice, notable for
good maternal instincts, were used as recipients for non-surgical
transfer of transgenic mouse embryos.
Animal husbandry
Transgenic rodent models were housed conventionally prior to the
rederivation. These animals received a non-sterilized diet (LASQCdiet®
Rod16-H, LASvendi, Germany) and untreated water ad libitum.
Water bottles and cages were changed once per week.
SPF animals were fed certified rodent diet (LASQCdiet® Rod18-H,
LASvendi, Germany) and autoclaved water was available
ad libitum. In the barrier facility, all cages, cage tops,
bedding and foodstuff went through peroxidase sterilization (Bioquell
Z- 2, Bioquell, UK) before use. Staff members entering the quarantine
suite followed all necessary precautions according to established SPF
guidelines and protocols.
Both conventional (embryo donors and breeding males) and SPF animals
(recipient females and vasectomized males) were maintained under
controlled environmental conditions according to good laboratory
practice requirements: at 22±2˚C with 55±10% relative humidity and a
12 h light/dark cycle (with the light phase starting at 7 am).
Vasectomized and breeding males were housed individually, donor and
recipient females were housed five per cage before being mated.
Ethical statement: This project was approved by the State
Veterinary and Food Committee of Slovak Republic (Ro 4429/16-221h; Ro
4429/16-221g; Ro 4429/16-221a) and by the Ethics Committee of the
Institute of Neuroimmunology at Slovak Academy of Sciences. All
procedures involving animals and their care were in accordance with
the approved guidelines conforming to international standards.
Embryo collection, culture and transfer
Mature wild-type females (2-4 months old) were naturally mated with
heterozygous transgenic males. Plug-positive dams were sacrificed by
cervical dislocation the following morning to obtain 1-cell embryos
(surgical method) or at Day 4 to obtain blastocysts (NSET method). For
surgical implantation in rats, 1- cell embryos were recovered from the
excised oviduct into M2 medium containing 0.1% hyaluronidase to remove
surrounding cumulus cells. After repeated washing (5 times) in sterile
media (M2), embryos were placed into M16 medium and cultured overnight
in an incubator (at 37˚C in 5% CO2) to reach 2-cell stage. The
following morning, the resulting embryos were washed again 5x in M2
media before being transferred unilaterally into the oviduct. Surgical
transfer was performed under general anesthesia using Zoletil 100®
(1:1 tiletamine-zolazepam mixture, 50mg/ml + 50mg/ml; Virbac, Carros,
France) in combination with Xylariem® (20mg/ml; Ecuphar N.V.,
Oostkamp, Belgium) administered intraperitoneally. At the end of
surgery, buprenorphine (0.1 mg/kg; Buprex; Reckitt Benckiser
Pharmaceuticals Ltd, Berkshire, UK) was administered intraperitoneally
to prevent postoperative pain. Following surgery, the animals were
placed inside clean cages warmed to 37°C until they regained
consciousness. The recipients were monitored regularly during next 2
days and received analgesics if required.
For non-surgical transfer in mice, embryos at blastocyst stage were
collected by flushing the uterine horns of donor females at 3.5 days
post coitum (dpc) and microscopically evaluated (Nikon SMZ645
stereoscopic Zoom Microscope, Prague, Czech Republic). Only
morphologically normal blastocysts with intact zona pellucida were
selected for subsequent transfer. Apart from repeated washing (5x) of
embryos in sterile media by using the new capillary for each washing
drop, no other pretreatment was used to reduce the concentration of
pathogens. Before each transfer, 6–8-week-old nulliparous CD-1 mice
(SPF animals) at proestrus were mated with vasectomized males to
induce pseudo-pregnancy. Plug-positive females were used as recipients
on 2.5 dpc. Briefly, each pseudopregnant mouse was placed on a
wire-top cage and allowed to grip the bars. A small speculum
(ParaTechs, Kentucky, USA) was inserted inside the vagina to open and
expose the cervix. The catheter then was inserted through the
speculum, past the cervical opening, and into the lumen of uterine
horn (Ali et al., 2014).
Embryo transfer procedures were repeated until a sufficient number of
transgenic founders was obtained. To evaluate the effectiveness of the
ET, the following parameters were analyzed: 1) pregnancy rate (% of
recipients becoming pregnant after ET); 2) birth rate (% of pups born
per embryos transferred); 3) transgenic rate (% of pups carrying
transgene per pups born). The success of the entire rederivation
procedure was determined based on criteria proposed by Van Keuren and
Saunders (2004) for transgenic lines.
Rederivation protocol
Two separate working groups were involved in the rederivation program.
Personnel at the conventional unit were responsible for embryo
isolation and initial washing with sterile M2 media (Sigma-Aldrich,
Bratislava, Slovakia) prior to delivery. For short-term transportation
(5-10 mins), donor embryos were placed into M2 medium in NUNC 4well
dish (Thermo Fischer scientific, Bratislava, Slovak Republic) at room
temperature and delivered directly to the SPF unit. Staff members at
the receiving SPF area repeated washing before transferring embryos to
clean surrogate mothers. All embryo transfers were performed within
30 min after embryo collection.
Genotyping and health monitoring
The offspring resulting from embryo transfers were tail tipped between
10-15 days of age. Genomic DNA samples from tail biopsies were
analyzed for the transgene expression by PCR assay using
transgene-specific primers mentioned earlier. Confirmed transgenic founders were used to establish
a clean breeding core of the respective strains under SPF
conditions. The next generation was obtained by natural mating of a
heterozygous transgenic male with a wild- type (WT) female. The
efficacy of the rederivation protocol was tested using sentinel
animals to monitor the microbiological state of an existing colony
(Reuter et al., 2003). Sentinels with the proven SPF state
were exposed to soiled bedding material from the cages of rederived
animals for at least 5 weeks. After this period, samples from the
sentinels were collected and outsourced to IDEXX BioAnalytics
(Germany) for microbiological testing.
Histological examination of brains
Animals from the subsequent generation of rederived lines (n=5/line)
were deeply anaesthetized with a mixture of Zoletil (30mg/kg) and
Xylazine and perfused transcardially with 4% paraformaldehyde (PFA)
in 0.1 M phosphate buffered saline, pH 7.2, using a mini peristaltic
pump (Harvard apparatus, USA). The tissues were post-fixed in 4 % PFA
after perfusion and then embedded in paraffin and cut on a microtome.
8µm paraffin embedded sections were immunostained with AT8 antibody
(epitope pS202/T205; Thermo Scientific, Illinois, USA). Following
washing, sections were stained with biotinylated secondary antibody
and developed using Vectastain ABC Kit (Vector Laboratories, CA, USA).
After mounting, sections were evaluated using an Olympus BX51 light
microscope equipped with an Olympus DP27 digital camera (Olympus,
Prague, Czech Republic). Stained sections were examined for presence
of AT8 positive structures.
Results
Rederivation of transgenic rats inside the SPF facility
We initiated our rederivation program in transgenic rat colonies in
which antibodies to Clostridium piliforme (formerly
Bacillus piliformis) were detected by MFI (Multiplex
Fluorescent Immunoassay) and confirmed by the indirect fluorescent antibody (IFA) test. Since
these transgenic animal models are not available commercially, it was
necessary to rederive them to safeguard their unique genotypes. The
well-established surgical ET method was employed for this purpose.
A total of 289 embryos were transferred into 24 pseudopregnant
recipients with an average (all embryos/ all recipients) of 12 embryos
transferred per unilateral oviduct. Of all recipients, 18 delivered
a total of 86 pups, out of which 36 were transgenic, with an
overall rate of 42% (Table 1).
Table 1. Results from the surgical implantations of transgenic rat lines
|
Transgenic rat line |
Number of recipients |
Number of pregnancies |
Number of embryos transferred |
Number of pups born |
Number of transgenic pups |
|
SHR72 |
6 |
5 (83%) |
77 |
17 (22%) |
6 (35%) |
|
SHR24 |
9 |
5 (56%) |
108 |
30 (28%) |
14 (47%) |
|
W72 |
9 |
8 (89%) |
104 |
39 (38%) |
16 (41%) |
|
Combined Total |
24 |
18 (75%) |
289 |
86 (30%) |
36 (42%) |
Import of transgenic mouse lines from conventional into a barrier
facility
The next step was to import transgenic mouse models from conventional
animal house into a specific pathogen free unit while simultaneously
eliminating murine pathogens:
Mycoplasma pulmonis, Klebsiella oxytoca and
Morganella morganii from breeding colonies. To accomplish
this goal, the non-surgical ET technique using the non-surgical embryo
transfer (NSET) device was selected. The reproductive data of
transgenic mouse lines following the NSET procedure are presented in
Table 2. A total of 109 in vivo-derived blastocysts were
transferred to 11 pseudopregnant CD1 recipients with an average of
about 10 blastocysts received per female. Of all recipients, 7 became
pregnant and gave birth to a total of 27 pups with an overall 48
% transgenic positive rate.
Table 2. Results from nonsurgical transfer of transgenic mice lines
|
Transgenic mouse line |
Number of blastocysts collected |
Number of blastocysts transferred |
Number of recipients |
Number of pregnancies |
Number of pups born |
Number of transgenic pups |
|
R3m/4 |
78 |
70 (89.7%) |
7 |
4 (57%) |
11 (15.7%) |
5 (45%) |
|
R3m/7 |
46 |
39 (84.8%) |
4 |
3 (75%) |
16 (41.0%) |
8 (50%) |
|
Combined Total |
124 |
109 (87.9%) |
11 |
7 (64%) |
27 (24.8%) |
13 (48%) |
Health monitoring of rederived colonies
A soiled bedding sentinel program was utilized to verify the health
status of the animals after rederivation. In this study, C57BL/6NCrl
or Crl: CD1 (ICR) mice (depending on their availability) and Crl: CD
(SD) rats were employed as sentinels. These animals were not screened
individually for infection prior to usage, but the health reports
declared that the source colonies were free of infection. For each
room, two sentinel cages (each stocked with two 4- to 6-week-old
females of a particular strain) were placed on the top or the bottom
of the rack to maximize the exposure to potential pathogens present in
the colony. Bedding samples from several randomly
selected cages were placed into each sentinel cage during weekly cage changing. After at least 5 weeks,
samples from sentinel animals were collected. These included blood for
serology testing, fecal samples for endoparasitology and PCR testing,
swabs from the respiratory tract as well as a fur swabs for an
ectoparasitology examination. As a result, the success of the
rederivation procedure was confirmed by the absence of all pathogens
listed in Tables 3 and 4 of FELASA recommendations (Mähler
et al., 2014).
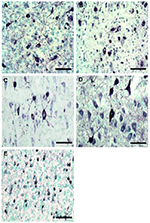
Neuropathological phenotype of rederived lines
The subsequent generation from rederived lines was obtained by
breeding (natural reproduction) under SPF animal facility conditions.
Interestingly, it has been observed that reproducibility of the
phenotype may be altered, in some cases, by relocating the animals
(Masopust et al., 2017). Therefore, in order to evaluate if
the rederived lines retained their phenotypes, we performed
histochemical staining of brainstem and cortex (SHR24) using
phospho-tau antibody AT8 (marker of neurofibrillary pathology). We
confirmed the presence of AT8 positive structures in transgenic mouse
(Fig.1 A-B) and rat (Fig. 1 C-E) models as previously reported
(Zilka et al., 2006; Stozicka et al., 2010; Filipcik
et al., 2012; Zimova et al., 2016).
|
Figure 1. Histological staining for neurofibrillary pathology
in rederived transgenic lines.
Immunohistochemical analysis using AT8 antibody revealed the
presence of neurofibrillary structures in (A) R3m/7, (B) R3m/4,
(C) W72, (D) SHR72 and (E) SHR24 transgenic lines. Brain
areas-A-D: Brainstem; E: Cortex. Scalebar: 100µm. Click image to enlarge |
Discussion
Transgenic rodents are used extensively to replicate different aspects of human diseases including critical aspects of the pathology of Alzheimer’s disease (Spires and Hyman, 2005; Zilka et al., 2006; Filipcik et al., 2012; LaFerla and Green, 2012; Zimova et al., 2016). However, most of these valuable rodent models are still being bred in conventional animal facilities. It is therefore essential to ensure that these animals are free of pathogens which may alter their unique phenotypes, and thus confound the interpretation of study results (Franklin, 2006; Treuting et al., 2011).
Our first challenge was to improve the health status of transgenic
rats and to generate colonies free of
Clostridium piliforme (formerly Bacillus piliformis)
in a fully occupied and endemically infected breeding facility. For
this purpose, we initiated the rederivation program through embryo
transfer using the standard surgical procedure. Since
Clostridium piliforme is an endospore-forming bacterium,
special efforts, including peroxidase sterilization and disinfection,
were made to remove spores from the environment. This procedure in
combination with successful embryo transfer resulted in the
elimination of the undesirable pathogen in rats. More importantly, we
were able to continuously use the facility without any emergency
shutdown or major renovation. We conclude that embryo transfer
rederivation coupled with the environmental decontamination can
eliminate latent infection caused by
Clostridium piliforme from transgenic rat colonies.
We then proceeded to import novel transgenic mouse models for
tauopathy from a conventional animal house into a barrier unit. The
introduction of new lines into SPF conditions demands strict
precautions in order to avoid entry of pathogens into the receiving
facility. Washing of embryos prior to transfer prevents the
transmission of the vast majority of prevalent pathogens, and thus
represents the most secure way to introduce potentially contaminated
animals into a particular hygienically defined area (Morell, 1999; Van
Keuren and Saunders, 2004; Fray et al., 2008; Amstislavsky
et al., 2013). Although there is a standardized washing
protocol for the embryos of livestock by passing them through 10 drops
of sterile media (Springfellow and Seidel, 1998), there are none for
rodent embryos (Mahabir et al., 2008). In the present study,
the in vivo derived blastocysts were washed five times and
only zona intact embryos were non-surgically transferred to SPF
recipients. None of the infectious diseases were transmitted from
infected donors to recipients via embryos, thus providing the evidence
that the washing procedures were done properly. Regarding the NSET
technique itself, we did not use large specula, as suggested by NSET
protocol, and used only smaller specula. A similar refinement to the
original protocol was made by Ali et al. (2014). On average about 10
blastocysts, obtained from naturally mated donor females with
heterozygous transgenic males, were transferred to each recipient.
Using the NSET method, we set up the rederivation protocol which
fulfils all criteria of the 3R concept (Reduction, Refinement and
Replacement). The NSET method provided the ability to quickly generate
live born offspring which substantially reduced the number of donors
and fosters needed. With the repeated use of the surrogate mothers
(Kolbe et al., 2012) together with the reduction of embryos
transferred, we could achieve the balance between effort and yield as
described by González-Jara et al. (2017). As a refinement
procedure, it allowed smooth atraumatic transfer of embryos that
avoided the trauma suffered by the recipient mice from surgical
intervention (Steele et al., 2013). Finally, the transport of mouse
lines as embryos, instead of living animals, is considered as
a partial replacement in the use of animals (Kelley, 2010).
We have previously reported that the expression of truncated tau
induced extensive neurofibrillary degeneration in the brainstem and
spinal cord of SHR72 and W72 rats (Zilka et al., 2006;
Stozicka et al., 2010), and in the cortex of SHR24 rats.
Similarly, R3m/4 mice developed tau pathology predominantly located in
the brainstem (Zimova et al., 2016). Interestingly, we have
observed that despite retaining the desired genotype, some animal
models did not develop robust pathology in the SPF barrier, when
compared to their conventionally housed counterparts (S. Jadhav,
unpublished observations). Furthermore, it was shown, that changes in
housing conditions, even within the same institution, can be
accompanied by loss of specific disease phenotype (Masopust
et al., 2017). Therefore we investigated whether the animal
models retained their neuropathological phenotypes following the
entire rederivation procedure. Our results confirmed that all
rederived animal models developed progressive neurofibrillary
degeneration in the examined brain area as previously reported (Koson
et al., 2008; Stozicka et al., 2010; Filipcik
et al., 2012; Zimova et al., 2016). The presence of
pathogens in donor animals, nor the movement of mouse embryos to
another facility, had a negative impact on their specific phenotype.
We conclude that all transgenic lines retained the neuropathological
phenotype following rederivation. Finally, our report, to the best of
our knowledge, is the first on rederivation of transgenic models of
human tauopathy.
Acknowledgements
We thank Jozef Végh, Jana Jergušová and Stanislava Mandáková for their technical help. We also thank Tomas Smolek for histological images. We thank the staff of the SPF animal facility for their excellent support. All authors approved to the final manuscript. The work is supported by grants APVV-14-0872, VEGA 2/0181/17, VEGA 2/0135/18 and Axon Neuroscience R&D Services SE.
Conflict of interest
The authors declared no potential conflict of interest.
References
- Amstislavsky, S.Y., Igonina, T.N., Rozhkova, I.N., Brusentsev, E.Y., Rogovaya, A.A., Ragaeva, D.S., Naprimerov, V.A., Litvinova, E.A., Plyusnina, I.F., Markel, A.L., 2013. Rederivation by embryo transfer in strains of laboratory mice and rats. Russian Journal of Genetics: Applied Research, 3(4), pp.305-315.
- Artwohl, J.E., Purcell, J.E., Fortman, J.D., 2008. The use of cross-foster rederivation to eliminate murine norovirus, Helicobacter spp., and murine hepatitis virus from a mouse colony. Journal of the American Association for Laboratory Animal Science, 47(6), pp.19-24.
- Bermejo-Alvarez, P., Park, K.E. , Telugu, B.P., 2014. Utero-tubal embryo transfer and vasectomy in the mouse model. Journal of visualized experiments, (84):e51214.
- Ali, R.B, van der Ahé, F., Braumuller, T.M., Pritchard, C., Krimpenfort, P., Berns, A., Huijbers, I.J., 2014. Improved pregnancy and birth rates with routine application of nonsurgical embryo transfer. Transgenic research, 23(4), pp.691-695.
- Chin, H.J., Wang, C.K.L., 2001. Utero‐tubal transfer of mouse embryos. Genesis, 30(2), pp.77-81.
- Du, Y., Xie, W., Liu, C., 2010. Strategies and considerations for distributing and recovering mouse lines. Methods in enzymology, 476, pp. 37-52.
- Filipcik, P., Zilka, N., Bugos, O., Kucerak, J., Koson, P., Novak, P., Novak, M., 2012. First transgenic rat model developing progressive cortical neurofibrillary tangles. Neurobiology of aging, 33(7), pp.1448-1456.
- Franklin, C.L., 2006: Microbial Considerations in Genetically Engineered Mouse Research. ILAR Journal, 47(2), pp.141–155.
- Fray, M.D., Pickard, A.R., Harrison, M., Cheeseman, M.T., 2008. Upgrading mouse health and welfare: direct benefits of a large-scale rederivation programme. Laboratory animals, 42(2), pp.127-139.
- González-Jara, P., Fontela, T., López-Mimbela, E., Cereceda, M., Del Olmo, D., Moreno, M., 2017. Optimization of the balance between effort and yield in unilateral surgical transfer of mouse embryos. Laboratory Animals, 51(6),pp622-628.
- Green, M.A., Bass, S., Spear, B.T., 2009. A device for the simple and rapid transcervical transfer of mouse embryos eliminates the need for surgery and potential post-operative complications. Biotechniques, 47(5), pp.919-924.
- Kelley, K.A., 2010. Transport of mouse line by shipment of live embryos. Methods in enzymology, 476, pp. 25-34.
- Kolbe, T., Palme, R., Touma, C., Rülicke, T., 2012. Repeated use of surrogate mothers for embryo transfer in the mouse. Biology of reproduction, 86(1), pp.1-6.
- Koson, P., Zilka, N., Kovac, A., Kovacech, B., Korenova, M., Filipcik, P., Novak, M., 2008. Truncated tau expression levels determine life span of a rat model of tauopathy without causing neuronal loss or correlating with terminal neurofibrillary tangle load. European Journal of Neuroscience, 28(2), pp.239-246.
- LaFerla, F.M., Green, K.N., 2012. Animal models of Alzeimer disease. Cold Spring Harbor Perspectives in Medicine, 2(11). pii: a006320.
- Le Monnier, A., Join-Lambert, O.F., Jaubert, F., Berche, P., Kayal, S., 2006. Invasion of the placenta during murine listeriosis. Infection and immunity, 74(1), pp.663-672.
- Mahabir E, Bauer B, Schmidt J (2008) Rodent and Germplasm Trafficking: Risks of Microbial Contamination in a High-Tech Biomedical World. ILAR Journal, 49(3):347-355.
- Mähler, M., Berard, M., Feinstein, R., Gallagher, A., Illgen-Wilcke, B., Pritchett-Corning, K., Raspa, M., 2014. FELASA recommendations for the health monitoring of mouse, rat, hamster, guinea pig and rabbit colonies in breeding and experimental units. Laboratory animals, 48(3), pp.178-192.
- Masopust, D., Sivula C.P., Jameson S.C., 2017: Of Mice, Dirty Mice, and Men: Using Mice to Understand Human Immunology. Journal of Immunology, 199(2),pp. 383–388.
- McLaren, A., Michie, D., 1956. Studies on the transfer of fertilized mouse eggs to uterine foster-mothers: I. Factors affecting the implantation and survival of native and transferred eggs. Journal of Experimental Biology, 33(2), pp.394-416.
- Morrell, J.M., 1999. Techniques of embryo transfer and facility decontamination used to improve the health and welfare of transgenic mice. Laboratory animals, 33(3), pp.201-206.
- Raspa, M., Mahabir, E., Fray, M., Volland, R., Scavizzi, F. 2016. Lack of transmission of murine norovirus to mice via in vitro fertilization, intracytoplasmic sperm injection, and ovary transplantation. Theriogenology, 86(2), pp.579-588.
- Reuter, J.D., Dysko, R.C., Reuter, J., Suckow, M.A., 2003. Quality assurance/surveillance monitoring programs for rodent colonies. Laboratory animal medicine and management, pp.1-13.
- Sarvari, A., Naderi, M.M., Sadeghi, M.R., Akhondi, M.M., 2013. A technique for facile and precise transfer of mouse embryos. Avicenna journal of medical biotechnology, 5(1), p.62.
- Sato, M., Watanabe, T., Kimura, M., 1999. Embryo transfer via oviductal wall: an alternative method for efficient production of transgenic mice. Transgenics, 2(4), pp.383-390.
- Shek, W.R., 2008. Role of housing modalities on management and surveillance strategies for adventitious agents of rodents. ILAR journal, 49(3), pp.316-325.
- Spires, T.L., Hyman, B.T., 2005. Transgenic models of Alzheimer’s disease: learning from animals. NeuroRx, 2(3), pp.423–437.
- Springfellow D.A., and Seidel S.M., 1998. Manual of the International Embryo Transfer Society. Intemational Embryo transfer Society, Illinois, United States, pp. 79–91.
- Steele, K.H., Hester, J.M., Stone, B.J., Carrico, K.M., Spear, B.T., Fath-Goodin, A., 2013. Nonsurgical embryo transfer device compared with surgery for embryo transfer in mice. Journal of the American Association for Laboratory Animal Science, 52(1), pp.17–21.
- Stozicka, Z., Zilka, N., Novak, P., Kovacech, B., Bugos, O., Novak, M., 2010. Genetic background modifies neurodegeneration and neuroinflammation driven by misfolded human tau protein in rat model of tauopathy: implication for immunomodulatory approach to Alzheimer´s disease. Journal of Neuroinflammation, 7:64.
- Suzuki, H., Yorozu, K., Watanabe, T., Nakura, M., Adachi. J., 1996. Rederivation of mice by means of in vitro fertilization and embryo transfer. Experimental Animals, 45(1), pp.33-38.
- Sztein, J.M., Kastenmayer, R.J., Perdue, K.A., 2011. Pathogen-free mouse rederivation by IVF, natural mating and hysterectomy. Advanced Protocols for Animal Transgenesis, pp. 615-642.
- Treuting, P.M., Clifford C.B., Sellers R.S., Brayton C.F. 2011. Of Mice and Microflora: Considerations for Genetically Engineered Mice. Veterinary Pathology, 49(1),pp. 44-63.
- Van Keuren, M.L., Saunders, T.L., 2004. Rederivation of transgenic and gene-targeted mice by embryo transfer. Transgenic research, 13(4), pp.363-371.
- Yeom, S.C., Yu, S.A., Choi, E.Y., Lee, B.C., Lee, W.J., 2009. Prevalence of Helicobacter hepaticus, murine norovirus, and Pneumocystis carinii and eradication efficacy of cross-fostering in genetically engineered mice. Experimental animals, 58(5), pp.497-504.
- Zilka, N., Filipcik, P., Koson, P., Fialova, L., Skrabana, R., Zilkova, M., Rolkova, G., Kontsekova, E., Novak, M., 2006. Truncated tau from sporadic Alzheimer’s disease suffices to drive neurofibrillary degeneration in vivo. FEBS letters, 580(15), pp.3582-3588.
- Zimova, I., Brezovakova, V., Hromadka, T., Weisova, P., Cubinkova, V., Valachova, B., Filipcik, P., Jadhav, S., Smolek, T., Novak, M., Zilka, N., 2016. Human truncated tau induces mature neurofibrillary pathology in a mouse model of human tauopathy. Journal of Alzheimer’s Disease, 54(2), pp.831-843.