Original scientific article
Improving well-being and survival in the 6-OHDA lesion model of Parkinson´s disease in mice: Literature review and step-by-step protocol
by Adriane Guillaumin, Bianca Vlcek and Åsa Wallén-Mackenzie*
Department of Organism Biology, Unit of Comparative Physiology,
Uppsala University
Correspondence: Åsa Wallén-Mackenzie, mail: asa.mackenzie@ebc.uu.se, Uppsala University, Department of Organism biology, Unit of Comparative Physiology, Norbyvägen 18 A, 752 36 Uppsala, Sweden
Summary
Parkinson’s disease (PD) is the most common neurodegenerative motor disorder and primarily affects movement control but also a range of non-motor functions. With unknown etiology and lack of cure, much research is dedicated to unravel pathological mechanisms and improve clinical prospects for symptom alleviation, prevention and treatment. To achieve these goals, animal models intended to represent symptoms similar to those observed in the complex clinical display of PD play a key role. It is important to bear in mind that, in any studies with laboratory animals, it is crucial to take the 3Rs principle (Refine, Reduce, Replace) into account. The main pathology of PD includes degeneration of dopamine neurons in the substantia nigra pars compacta (SNc). The 6-hydroxydopamine (6-OHDA) lesion model, in which dopaminergic neurons are chemically destroyed, is often favored as a laboratory model of PD in both rodents and primates. However, while reproducing several features of clinical PD, mice exposed to 6-OHDA frequently experience systemic dysfunction causing premature death. To avoid suffering and unnecessary deaths of laboratory mice, there is a need for improved experimental protocols in accordance with the 3Rs principle. Based on current literature and our own previous experiments, we decided to test the effect of three parameters: 1) reduced dose of the 6-OHDA toxin; 2) daily post-operative care to avoid hypothermia and energy loss; 3) shortened interval from surgical injection of toxin to time of sacrifice.
By implementing a 6-OHDA lesion protocol using a lower dose of toxin than commonly seen in the literature alongside careful post-operative care and decreased time post-injection, a fully recovered weight post-surgery and high survival rate was obtained. This was achieved despite full expression of the 6-OHDA-induced locomotor phenotype. A step-by-step protocol was formulated. Validation using histological analysis confirmed toxin-induced degeneration of midbrain dopamine neurons with concomitant loss of dopaminergic projections in the lesioned hemisphere. Notably, while SNc dopamine neurons were drastically reduced, those located in the ventral tegmental area (VTA) were less affected in a medialhigh survival to laterallow survival manner.
Introduction
Parkinson’s disease (PD) is the second most common progressive neurodegenerative disease with a prevalence of 1 to 2 per 1000 (Khan et al. 2019; Obeso et al. 2017; Tysnes and Storstein 2017). PD patients are usually diagnosed around the age of 65 years, when motor symptoms begin to appear. Patients suffer from a progressive slowness in initiating movement, loss of postural reflexes and, with time, a rigidity of muscles. Some, but not all, PD patients also display resting tremor. A characteristic hallmark commonly observed in the brain of PD patients is the presence of so called Lewy bodies, accumulations of a misfolded protein known as α -synuclein (Barker and Williams-Gray 2016; Braak et al. 2003; Schneider and Obeso 2015; Spillantini et al. 1997). Life expectancy is shorter in PD, and many patients experience much suffering due to a broad range of symptoms beyond voluntary motor control, including bladder control, pain, depression and cognitive dysfunction, such as dementia (Armstrong and Okun 2020; Chaudhuri et al. 2006; Kalia and Lang 2015; Park and Stacy 2009; Pfeiffer 2016).
A main pathology of PD is the progressive degeneration of midbrain
dopamine (DA) neurons, primarily those located in the substantia nigra
pars compacta (SNc) (Kordower et al. 2013). DA neuron
pathology is a key aspect of the clinical diagnosis of PD as it can be
determined by brain imaging analysis such as PET scan (Rispoli et al.
2018). With time, DA neurons of the ventral tegmental area (VTA), that
usually show higher resilience to PD, might also degenerate. Loss of
VTA DA neurons is often associated with affective and cognitive
dysfunction. As the disease progresses, multiple brain systems are
affected which leads to an even further increase in symptoms and level
of suffering for the patient (Muñoz et al. 2020; Paredes-Rodriguez et
al. 2020). Still today, there is no cure for PD.
SNc DA neurons are of particular interest in both clinical and
pre-clinical (experimental) research. These neurons project primarily
to the caudate putamen, mostly referred to as the dorsal striatum in
rodents, forming the nigrostriatal pathway. This pathway is a critical
modulator of motor control via the basal ganglia and associated
structures (caudate putamen (striatum), globus pallidus and the
subthalamic nucleus (STN)) (Graybiel 2000; Sesack and Grace 2010). SNc
DA neurons extert a strong impact on movement via the so called direct
and indirect pathways of the basal ganglia, where nigrostriatal DA
release facilitates movement initiation in normal conditions. In
contrast, in the parkinsonian brain, the degeneration of DA neurons in
the SNc leads to a progressive loss of DA release in the striatum
which in turn causes difficulty in movement initiation via the basal
ganglia (Björklund and Dunnett 2007). When the patient becomes aware
of movement difficulties, such as difficulty in initiating walking, as
much as 50-90% of SNc DA neurons have already been lost (Kordower et
al. 2013). Understanding the pathological process of PD, not least to
find a cure, but also to allow earlier detection and to enable
preventive strategies, is an important goal in PD research (Obeso et
al. 2017). In terms of treatment, much effort has been aimed at
replacing the loss of DA, either by pharmacological administration of
DA or its agonists, or by DA cell replacement. Clinically, the
administration of DA agonists along with inhibitors of monoamine
oxidase B activity is often used in early stages of PD to improve
motor function (Armstrong and Okun 2020; Connolly and Lang 2014).
Another important treatment is the administration of levodopa
(L-dopa), a precursor to DA, which is highly efficient in alleviating
motor symptoms in PD (LeWitt 2015). However, after prolonged use and
with the progressive degeneration of neurons, L-dopa administration
has the unfortunate side-effect of inducing dyskinesias (Olanow and
Stocchi 2018; Yang et al. 2021). Much research is therefore aimed at
reducing these side-effects by, for example, implementing continuous,
rather than intermittent, L-dopa administration (Senek and Nyholm
2014). Cell replacement is another strongly emerging research field
aiming at replacing the degenerated DA neurons with newly generated
ones derived from various types of stem cells (Cyranoski 2018;
Grealish et al. 2014; Parmar et al. 2020). Yet another type of PD
treatment, which is already clinically implemented with a high success
rate, is so called deep brain stimulation (DBS). DBS consists of
high-frequency electrical stimulation delivered via implanted
electrodes positioned in brain areas in which the firing activity has
been altered as a consequence of the brain pathology in PD. The two
most common brain targets for DBS electrodes in PD are the STN and the
globus pallidus, both of which display aberrant activity in PD
(Alexander et al. 1990; Eisinger et al. 2019). While providing
successful alleviation of motor symptoms, the mechanisms underlying
DBS have remained largely unknown (Chiken and Nambu 2016; Herrington
et al. 2016; Miocinovic et al. 2013). Further, DBS treatment has in
some cases been the cause of adverse behavioral and cognitive
side-effects, the reasons of which remain to be discovered (Accolla
and Pollo 2019; Benabid et al. 1994; Heywood and Gill 1997; Kim et al.
2015; Petry-Schmelzer et al. 2019).
Taken together, it is evident that much more knowledge is needed to
fully understand the pathological changes underlying PD, both to find
new opportunities for prevention and treatment, and to optimize
current invervention strategies. Due to the complexity of PD, which
affects multiple brain circuits with broad impact on the affected
individual´s well-being, whole-animal experimental approaches are
crucial. Animal models of PD are commonly based either on gene
mutations that have been observed in cohorts of PD patients (e.g.
LRRK2, α-synuclein (Dawson et al. 2010)), pharmacological intervention
(e.g. reserpine, haloperidol (Lorenc-Koci et al. 1996)), or
neurotoxins (e.g. N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, MPTP;
6-hydroxydopamine, 6-OHDA (Hernandez-Baltazar et al. 2017; Kin et al.
2019)).
Among these experimental PD models, the 6-OHDA lesion model is
commonly used when aiming to induce DA neuron degeneration (Boix et
al. 2015; Cenci and Björklund 2020; Park et al. 2018; Thiele et al.
2012). The 6-OHDA molecule resembles DA and is taken up by the
endogenous cytoplasmic membrane transport machinery present in all
catecholaminergic neurons. Inside the cell, two mechanisms of action
of 6-OHDA are believed to cause cell death: disturbance of the
mitochondrial respiratory chain and auto-oxidation of the 6-OHDA
molecule which leads to the production of reactive oxygen species
(Blum et al. 2001; Glinka et al. 1997; Hernandez-Baltazar et al.
2017). In the experimental setting, 6-OHDA solution is usually
injected unilaterally to allow one side of the brain to remain
undisturbed by the deleterious effects of the toxin. Each animal
thereby has one hemisphere with an intact midbrain DA system, serving
as an internal control, and one hemisphere with a 6-OHDA-lesioned DA
system. Upon unilateral injection of 6-OHDA, into either the SNc or
the nigrostriatal pathway in the median forebrain bundle (MFB), the
rodent displays rotational behavior due to loss of movement control by
the lesioned hemisphere. The effect of 6-OHDA-induced lesion is fast,
which is beneficial for an acute animal model, but with the drawback
of a lack of slowly progressive cell loss which is a core feature of
clinical PD. The efficiency of the 6-OHDA-induced lesion can be
ascertained in each animal by assessing rotational movement, and its
exacerbation by a DA-releasing agent, such as the psychostimulant
amphetamine, while the extent of DA neuron degeneration requires
post-mortem brain analysis, usually by the use of histological markers
for DA neurons.
The 6-OHDA lesion model is today a classical and frequently
implemented model to generate subtypes of symptoms representative of
PD in experimental animals, including both rodents and primates. Mice
are particularly useful for a range of viral-genetics based
experiments due to the abundance of transgenic lines available today
which allow chemogenetic and optogenetic control over neuronal
activity. The possibility to combine these powerful methods with
disease models provides great potential for improving knowledge of how
to predict, prevent and treat difficult disorders. This is indeed the
case for PD for which animal-based experiments have been essential for
the knowledge available today. However, mimicking human disorders in
mice is difficult, and toxicity-based models, while replicating
several human symptoms, risk harming the animal to an extent that
outweighs any gain the model can possibly bring in terms of new
knowledge. When it comes to implementing the 6-OHDA lesion model in
experimental mice, ethical concerns are therefore raised due to the
frequent appearance of post-surgical weight-loss and premature death.
Lesioned mice experience difficulty in both feeding and maintaining
body temperature (Boix et al. 2015; Masini et al. 2021). Ethical
considerations commonly grade the 6-OHDA lesioning in mice as a severe
procedure. In accordance with the 3Rs principle (Refine, Reduce,
Replace) in experimental animal welfare (European Union 2010; Percie
du Sert et al. 2020; Tannenbaum and Bennett 2015), one crucial aspect
of using laboratory animals is to strive for protocols that fulfil the
Refine and Reduce criteria, minimizing suffering and using sufficient
animals to draw relevant and useful conclusions but without
sacrificing unneccessary animals.
With our interest in generating an improved protocol for the 6-OHDA
lesion model in laboratory mice, we searched the literature for
standard doses used in rodents, common time post-lesioning until
sacrifice, and procedures of post-operative care. Based on the
findings, to create conditions with high survival rate and well-being
for 6-OHDA lesioned mice while maintaining efficient degeneration of
the midbrain DA system, three refinements were made to the protocol:
1) reduced dose of the toxin; 2) careful post-operative care; 3)
reduced post-lesioning time. Analysis of several cohorts of mice
demonstrates that these precautions lead to the expected toxin-induced
loss of midbrain DA neurons, but instead of premature death, mice
recover well, re-gain weight and show high survival rate. Finally,
using a range of DA cell markers, it could be concluded that loss of
DA neurons had taken place after two weeks, which makes it possible to
shorten the post-operative time substantially. This study contributes
to an experimental protocol in which animal safety has been taken into
consideration, by outlining detailed improvements in the commonly used
6-OHDA lesion model in laboratory mice.
Materials & Methods
Animals and groups
Adult C57BL/6NTac (Taconic) male mice (> 8 weeks old) were used to
generate and validate a 6-OHDA parkinsonian model. Mice were bred
in-house and housed at the animal facility of Uppsala University in
GM500 cages (Tecniplast, Italy). Each cage contained bedding (Tapvei,
Estonia), nesting (shredded paper) and enrichment (carton house)
materials (Brogaarden, Denmark). Mice had access to food pellets
(Lantmännen, Sweden) and tap water ad libitum in standard
humidity (45% relative humidity) and temperature (22°C ±2°C)
conditions. The housing room was set on a 12-hour dark/light cycle
(light on 6:00 to 18:00) with 30 minutes dawn/nightfall transitions.
All animal experimental procedures followed Swedish (Animal Welfare
Act SFS 1998:56) and European Union Legislation (European Union 2010).
Three groups of mice are reported (Group#0, Group#1, Group#2). A pilot
experiment (referred to as Group#0) is included in this report in
which mice received a commonly reported dose of 6-OHDA (2.8 and 3.0
mg/mL free base 6-OHDA; N=6). However, due to premature death, this
experimental setup was terminated. The full experimental group
(Group#1) instead received 1.85 mg/ml free-base 6-OHDA. Group#1 was
divided in one cohort of mice sacrificed two weeks (14 days) after
lesioning and a second cohort sacrificed three weeks (21 days) after
lesioning. Within each cohort, mice were divided into a 6-OHDA
subcohort and a control (vehicle) subcohort. Littermate mice were
randomly assigned to the different cohorts and sub-cohorts with a
maximum of 5 mice per cage. To complement this experimental group
(Group#1), a second batch of mice (Group#2) received 1.85 mg/ml
free-base 6-OHDA and was sacrificed two weeks (14 days) after
lesioning.
Group#1: N=18. N=11 6-OHDA injected mice: 9 mice for
in situ hybridization analysis (N=8 after the death of one
mouse) and 2 mice for immunohistochemistry analysis; N=7 control mice
for in situ hybridization analysis. Cohorts and subcohorts
for in situ hybridization analysis: Two weeks post-lesion,
N=4 6-OHDA mice, N=4 control mice. Three weeks post-lesion: N=4 6-OHDA
mice, N=3 control mice.
Group#2: N=11 6-OHDA injected mice, sacrificed two weeks post-lesion
(N=9 after the death of two mice).
N total (Group#1 + Group#2) = 29 of which N= 22 6-OHDA and 7 controls
(vehicle).
Pre-operative care
Two days prior to 6-OHDA injection, sunflower seeds were provided in
the home cage to increase body weight and habituate the mice to this
food supplement.
6-OHDA lesioning
Mice were anesthetized with a mix of air and isoflurane (4 L/min
isoflurane-air mix v/v) and maintained anesthetized with 0.5-2 L/min
of isoflurane. Mice were then placed in a stereotaxic apparatus and
injected subcutaneously with an anti-inflammatory drug (Carprofen,
5mg/mL; Norocarp). After subcutaneous injection of a topical analgesic
(Marcain, 1.5 mg/kg; AstraZeneca), the skin was incised to expose the
skull. A hole was drilled above the MFB. Coordinates on the right
hemisphere: antero-posterior: -1.20 mm from bregma and medio-lateral:
-1.10 mm from the sagittal vein. The 6-OHDA solution was freshly
prepared on the day of injection by dissolving 2.2 mg of
6-OHDA-hydrochloride in 0.9% NaCl / 0.02% ascorbic acid solution
(vehicle) and protected from the light. For control mice, 1 µL of the
vehicle was injected. 1 µL of 6-OHDA solution (1.85 mg/mL) or vehicle
was injected with a NanoFil syringe (World Precision Instruments,
Sarasota, FL, USA) at 100 nl/min, at -4.75 mm from the brain’s
surface. After 10 min, the needle was slowly removed and the skin
stitched. Mice received another injection of Carprofen 20-24 hours
after the surgery. A step-by-step protocol is provided in Additional
File 1.
Post-operative care
Following surgery, the weight of the mice was monitored daily. Mice
administered with 6-OHDA received subcutaneous injections of
pre-heated saline (35-37°C, 1 mL) every day until sacrifice to prevent
dehydration and hypothermia. In addition, to provide easily accessible
food in the home-cage environment, food pellets immersed in a 15%
sucrose solution were placed on the floor of the home cage as well as
a petri dish filled with the same sucrose solution. Sunflower seeds
were placed in the cage a couple of days prior surgery and after
surgery until sacrifice. Nutritionally fortified water gel was also
placed in the home-cage for easy access to prevent dehydration and
allow weight preservation and/or gain.
Tissue preparation
Two or three weeks after 6-OHDA injections mice were sacrificed by
cervical dislocation. Brains intended for histological analysis by
in situ hybiridzation were frozen in -30 to -35°C
2-methylbutane (≥99%, Honeywell) and stored at -80°C until sectioned
with a cryostat (Leica Microsystems, Germany). For histological
analysis by immunohistochemistry, whole-animal perfusion with 1X
phosphate buffer saline (PBS) followed by ice-cold 4% formaldehyde
(FA) was performed, whereupon brains were extracted, post-fixed in 4%
FA for 24 hours and cut with a vibratome (Leica Microsystems,
Germany).
Immunohistochemistry
60 µm-thick coronal brain sections were washed and incubated for 15
min in a 1% hydrogen peroxidase solution diluted in 1X PBS 0.3%
TritonX-100 (0.3% PBST) at room temperature (RT). After rinsing,
sections were incubated for 90 min in a blocking solution containing 5
% normal donkey serum (NDS, Millipore) and 0.3% PBST. Sections were
then incubated with a primary antibody, rabbit anti-tyrosine
hydroxylase (anti-TH, 1:4000, AB152, Millipore), diluted in 0.3% PBST
and 1% NDS overnight at 4°C. The next day, sections were rinsed in 1X
PBS and then incubated with a secondary antibody, donkey anti-rabbit
biotin-SP-conjugated (1:1000, AP182B, Millipore), diluted in 0.3% PBST
for 90 min at RT. After washes, an avidin/biotin-based peroxidase
system (Vector ABC kit PK-6100, Vector Laboratories) was used to
amplify the signal for 90 min followed by a pre-wash in Tris 0.1 M
pH7.4 for 10 min. Sections were incubated in a DAB peroxidase solution
(SK-4100, Vector Laboratories). Sections were then washed in Tris 0.1
M pH7.4 for 10 min followed by two more washes in 1X PBS. The sections
were mounted on glass slides, counterstained with cresyl violet,
dehydrated with increasing concentrations of ethanol and mounted with
a mounting medium (Leica CV Mount). Sections were scanned with
NanoZoomer 2-0-HT.0 and visualized with NDP.view2 software
(Hamamatsu).
Fluorescent in situ hybridization
16 µm-thin coronal sections were cut using the cryostat and mounted on
glass slides for fluorescent in situ hybridization, FISH.
To allow detection of two mRNAs in the same section, the protocol
outlined below (and fully available in (Viereckel et al. 2016)) was
implemented. FISH experiments were performed to detect the following
mRNAs: Aldehyde dehydrogenase 1 (Aldh1a1), Calbindin1 (Calb1),
Gastrin-releasing peptide (Grp) and Vesicular monoamine transporter 2
(Vmat2), all co-analyzed with Th mRNA. Probe design was made by
Oramacell and described previously (Dumas and Wallén-Mackenzie 2019).
The mRNA sequences detected by the riboprobes were: Th: NM_012740.3;
bases 456-1453. Vmat2: NM_013031.1; bases 700-1440. Calb1:
NM_009788.4; bases 79-870. Grp: NM_175012.4; bases 127-851. Aldh1a1:
NM_001361503.1; bases 1895-2893.
Sections were thawed and post-fixed in 4% paraformaldehyde (4% PFA)
for 10 min at RT. After washes in 1X PBS, sections were incubated in a
triethanolamine solution (TEA, pH 8) for 5 min followed by incubation
in acetic anhydrade-containing TEA solution. Riboprobes (Digoxigenin
(DIG)-labelled riboprobe, 50-75 ng/100 µl and fluorescein-labelled
riboprobe, 75-100 ng/100 µl) were denatured in hybridization buffer at
85°C for 10 min and applied on the sections for 16-18 hours incubation
at 65°C in a humidified chamber. After hybridization, sections were
washed in 65°C saline sodium citrate buffer (SSC) baths (5X SSC
followed by 0.2X SSC solutions) and a last wash with a 0.2X SSC at RT.
Sections were then washed in 1X maleic acid buffer containing Tween20
(MABT) to decrease the binding of non-specific probes. Fluorescein
riboprobe was revealed by incubating the sections in a blocking
solution containing a blocking reagent (BR, Roche), heat-inactivated
FBS and 5X MAB. Sections were then incubated with an anti-fluorescein
horseradish peroxidase conjugated (POD) antibody (Roche) for 1 hour.
An amplification step was performed by incubating the slices in
TSA-biotin amplification buffer (Perkin Elmer) for 15 min.
Fluorescein-TSA-biotin complex was revealed by adding
Neutravidin-Oregon Green (Invitrogen) for 15 min. After rinsing the
sections in Tween20 PBS (PBST), hydrogen peroxidases were inhibited by
incubation in 0.1M glycine (pH 2.1) and 3% hydrogen peroxide (H2O2). A
similar procedure was followed to subsequently reveal the DIG
riboprobe with an anti-DIG-POD antibody diluted in BR solution and TSA
buffer plus Cy3 (Perkin Elmer). Sections were incubated for 10 min in
1/50000 DAPI solution and mounted with Fluoromount (Southern Biotech).
Slices were scanned with NanoZoomer 2-0-HT.0 scanner using the
NDP.scan 3.3 software (Hamamatsu).
Quantitative analysis of the DA markers was made by manually comparing the mRNA positive cells between the 6-OHDA lesioned side with the intact side. A similar amount of mRNA positive cells on the 6-OHDA lesioned side and the intact side gives a percentage of 100%. A lower percentage indicates decreased gene expression on the lesioned side, in comparison to the intact side. Percentages are indicated by : (-) No neurons or extremely few neurons; (+) More than a few neurons, up to 30% of the intact side; (++) 30-50% compared to intact side; (+++) 50-80% compared to intact side; (++++) More than 80% compared to intact side; (NA) denotes Not applicable: for areas where the mRNA is lacking in the control, in accordance with literature.
Table 1: Summary of current literature on 6-OHDA protocols implemented in experimental mice and rats. NS: not specified; MFB: median forebrain bundle; SNc: substantia nigra pars compacta; VTA: ventral tegmental area; E1: experiment 1; E2: experiment 2; E3: experiment 3; C1: concentration 1; C2: concentration 2; C3: concentration 3; C4: concentration 4; s.c.: subcutaneous; i.p.: intraperitoneal.
|
Mouse strain |
Sex |
Concentration of free-base |
Volume injected |
Injection site |
Survival rate |
Pre- and post-operative care |
Reference |
|
Current study: |
|||||||
C57BL/6NTac |
Group#0 (N=6)
Group#1
Group#1 + Group#2 |
2.8 and 3.0 mg/ml
1.85 mg/ml
1.85 mg/ml |
1 µl
1 µl
1 µl |
MFB
MFB
MFB |
50%
90.9%
86.4% |
Pre-operative care:
Post-operative care:
|
Guillaumin, Vlcek, Wallén-Mackenzie (current study) |
|
Literature review: |
|||||||
|
C57BL/6J and Vgat-IRES-Cre (Slc32a1) |
Male and female |
4.44 mg/ml |
1 µl
|
MFB
|
NS |
Pre-operative care: NS Post-operative care:
|
Bichler et al. 2021 |
C57BL/6 |
Male and female |
4 µg/µl |
1 µl
|
Striatum (bilaterally) |
95% |
Pre-operative care:
Post-operative care:
|
Masini et al. 2021 |
C57BL/6J |
Male and female |
15 µg/µl |
0.2 µl |
MFB |
96% |
Pre-operative care:
Post-operative care:
|
Koski et al. 2019 |
C57BL/6 |
Male |
15 mg/ml |
0.2 µl |
MFB |
80% |
Pre-operative care: NS Post-operative care:
|
Rentsch et al. 2019 |
C57BL/6 |
NS |
0.5 µg/µl (C1) 1 µg/µl (C2) 2 µg/µl (C3) 4 µg/µl (C4) |
1 µl
|
MFB |
NS |
Pre-operative care: NS Post-operative care: NS
|
Park et al. 2018 |
C57BL/6 |
Male |
NS |
NS |
Striatum |
NS |
Pre-operative care: NS Post-operative care: NS |
Im et al. 2016 |
C57BL/6J |
Male |
3.6 mg/ml |
1 µl |
MFB |
NS |
Pre-operative care: NS Post-operative care:
|
Sanders and Jaeger 2016 |
129P2/OLA Hsd |
Female |
4 g/l |
1 µl
|
MFB Striatum |
NS |
Pre-operative care: NS Post-operative care:
|
Bagga et al. 2015 |
C57BL/6 |
Male |
3mg/ml |
2 x 2 µl |
Striatum |
NS |
Pre-operative care: NS Post-operative care: NS |
Stayte et al. 2015 |
|
Rgs5gfp/+ |
Male |
0.3 µg/µl (C1) 0.7 µg/µl (C2) 1 µg/µl (C3) 3.6 µg/µl (C4) |
1 µl |
MFB |
91% (C1, C2 and C3) 80% (C4) |
Pre-operative care: NS Post-operative care:
|
Boix et al. 2015 |
C57BL/6J |
Male |
4 µg/ul |
1 µl |
Striatum (bilaterally) |
NS |
Pre-operative care: NS Post-operative care: NS |
Bonito-Oliva et al. 2014 |
Swiss Albino |
Male |
20 µg/µl |
3 µl |
Cerebral ventricle |
50% |
Pre-operative care: NS Post-operative care: NS |
Ribeiro et al. 2013 |
C57BL/6 |
Male |
6 µl/µg |
1 µl 2 x 1.5 µl 1.5 µl
|
MFB Striatum SNc |
83.3% 95% >90% |
Pre-operative care: NS Post-operative care:
|
Heuer et al. 2012 |
FVB |
NS |
15 mg/ml |
V = 0.2 µl |
MFB |
NS |
Pre-operative care: NS Post-operative care:
|
Thiele et al. 2012 |
C57BL/6 |
NS |
3.2 µg/µl |
1 µl 2 x 1 µl 2 x 2 µl 1 µl |
MFB Striatum Striatum SNc |
E1: 80% E1: >90% E1: 80% E1: >90%
E2 and E3: |
Pre-operative care: NS
Post-operative care: E2 and E3:
|
Francardo et al. 2011 |
NMRI |
Female |
1.6 µg/µl |
1.5 µl |
SNc |
NS |
Pre-operative care: NS Post-operative care: NS |
Grealish et al. 2010 |
C57BL/6 |
Male |
3.0 µg/µl |
1 µl 2 x 2 µl |
MFB Striatum |
14% 65% |
Pre-operative care: NS Post-operative care: NS |
Lundblad et al. 2004 |
|
Rat strain |
Sex |
Concentration of free-base |
Volume injected |
Injection site |
Survival rate |
Post-operative care |
Reference |
|
Athymic nude and Sprague-Dawley rats |
Male and female |
NS |
NS |
MFB |
NS |
Pre-operative care: NS Post-operative care: NS |
Grealish et al., 2014 |
Albino Wistar rats |
Male |
4 µg/µl 4 µg/µl
6.7 µg/µl |
4 µl
4 µl + 3 µl |
MFB MFB + VTA Striatum |
NS NS
NS |
Pre-operative care: NS Post-operative care: NS |
Yuan et al. 2005 |
Sprague–Dawley rats |
Male |
7.5 µg/ul |
1 µl |
MFB |
NS |
Pre-operative care: NS Post-operative care: NS |
Tan et al. 2000 |
Results
Well-being and high survival rate confirmed upon implementation of a
low dose 6-OHDA toxin followed by daily post-operative care to
prevent dehydration and hypothermia
As reported in the literature, the concentration of 6-OHDA commonly used to create parkinsonian locomotor symptoms in rodents varies from 2.5 to 3.6 mg/ml free-base (Bagga et al. 2015; Francardo et al. 2011; Masini et al. 2021; Sanders and Jaeger 2016). This concentration range tends to cause variable well-being and survival (for literature review, see Table 1). In accordance with published literature, we first tested the doses 2.8 and 3.0 mg/mL in a small group of C57BL/6NTac mice (Referred as Group#0; Table 1). However, the mice did not cope well after the injection of 6-OHDA (Figure 1C, weight curve of Group#0). Only 50% survived, and because they showed weight loss, inactivity and loss of appetite, the mice were sacrificed to avoid suffering (Figure 1C). Instead, we decided to test a lower dose to improve the protocol, and therefore reduced the concentration to 1.85 mg/ml free-base 6-OHDA.
A cohort of 18 C57BL/6NTac mice (Group#1) was injected with either
6-OHDA or vehicle into the MFB of the right hemisphere and mice were
subsequently divided into two groups depending on the duration of
protocol, two or three weeks until sacrifice (Figure 1A and Figure
2A). Frequent post-operative side-effects of the 6-OHDA lesioning
procedure include dehydration and hypothermia, both of which can be
prevented by special care (Boix et al., 2015; Francardo et al., 2011;
Table 1). In our experiment, pre-heated saline was therefore injected
daily which prevented hypothermia after surgery. Also, for easy
access to palatable food and water, sucrose-immersed food pellets and
a petri dish filled with sucrose solution were placed on the floor of
the home-cage. This enabled the mice to drink and feed without the
need for caretaker handling which prevented dehydration and allowed
weight gain (Figure 1A-C, Table 1). In accordance with the literature,
nutritional supplementation in the form of sunflower seed was provided
both pre-and post-surgery, and also gel-embedded nutrients were
provided post-surgery (Boix et al., 2015; Felgenhauer et al., 2020;
Lecker and Froberg-Fejko, 2016; Rentsch et al., 2019; Thiele et al.,
2012; Table 1). Initial weight loss was observed in the first days and
a peak of weight loss was reached at day 7 (26%) (Figure 1B-C).
However, on subsequent days, body weight rapidly increased resulting
in an average weight loss below 15% from day 11 and below 5% at day 15
(Figure 1B-C, Table 1). At 21 days post-surgery, when terminated for
analysis, Group#1 mice had recovered their weight. After the analysis
of Group#1, a second group (Group#2) was injected with 6-OHDA
following the same protocol as for Group#1. The weight curve for
Group#2 was similar to Group#1. However, Group#2 showed even less
weight loss than Group#1 with a peak of weight loss at day 6 (15%
average weight loss) and a progressive recovery on the subsequent days
(Figure 1C, Table 1). The general well-being of the mice surviving
treatment with the improved 6-OHDA lesion protocol (Group#1, Group#2)
can therefore be considered to have been satisfactory.
All control mice survived (100% survival). Three of the 22 mice
injected with 6-OHDA died before the day of sacrifice (one mouse in
Group#1 and two in Group#2; Table 1). The survival rate was thereby
86.4% among all 22 6-OHDA-treated mice, and 90.9% among the 11 mice in
Group#1 that were processed for histological analysis, see below and
Table 1.
A step-by-step protocol for the preparation of mice for surgery and
the surgical procedure as well as post-operative care according to the
details above is provided for easy download and implementation (Additional File 1).
Validation of the 6-OHDA lesion protocol: Mice displayed
spontaneous rotations and the midbrain dopamine system had
degenerated in the lesioned hemisphere.
Despite the comparatively low dose of 6-OHDA injected (1.85 mg/ml
free-base 6-OHDA), all 22 mice displayed spontaneous ipsilateral
rotations, starting directly after the surgery and lasting until
sacrifice. The spontaneous rotations functionally confirmed the
successful lesion of the nigrostriatal pathway using the injection
concentration of 1.85 mg/ml free-base 6-OHDA. Next, to validate the
extent of degeneration of DA neurons caused by the induced lesion,
brains were analyzed by histological methods.
First, tyrosine hydroxylase (TH), the rate-limiting enzyme in DA
synthesis and the most common marker used to visualize midbrain DA
neurons and their projections, was analyzed by immunohistochemistry.
For each mouse, the 6-OHDA-lesioned side was compared with the
non-lesioned side, each animal thus serving as its own control.
Compared to the non-lesioned side, the lesioned side of the brain
showed a strongly reduced amount of cells positive for
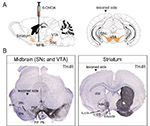
TH-immunoreactivity (IR) in the SNc and VTA, demonstrating that DA
neurons had degenerated as a consequence of the 6-OHDA-injection
(Figure 2B,
Supplementary figure 1).
Primarily DA neurons of the SNc were affected by the 6-OHDA injection
with very few TH-IR-positive neurons detected on the lesioned side
(Figure 2B,
Supplementary figure 1). In the VTA, TH-IR was only partially affected (Figure 2A,
Supplementary figure 1). The medial nuclei of the VTA, corresponding
to the interfascicular nucleus (IF), rostral linear nucleus (RLi) and
the medial parts of the paranigral nucleus (PN) and
parainterfascicular nucleus (PIF), remained largely intact. In
contrast, TH-IR was strongly reduced in the ventral and lateral nuclei
of the VTA of the lesioned side. These nuclei correspond to the
lateral part of the parabrachial pigmented nucleus (PBP) and
ventro-lateral parts of the PN and PIF (Figure 2B, Supplementary
figure 1). Thus, VTA neurons located closest to the SNc were more
strongly affected by 6-OHDA than those VTA DA neurons located further
away from the SNc and thus closer to the midline. In the VTA, DA
neurons thereby showed a medialhigh survival to laterallow survival
response after 6-OHDA lesioning using the current protocol (Figure
2B).
When addressing TH-positive projections from midbrain DA neurons, a
strong decrease throughout the length of the nigrostriatal pathway was
observed on the lesioned side. This directly matched the decrease of
TH-positive neurons observed in the ventral midbrain (Figure 2B). No
TH-positive fibres were visible on the lesioned side in the primary
target area of SNc DA neurons, the dorsal aspect of the striatum
(dStr) (Figure 2B). VTA neurons project in a topographical manner to
the ventral aspect of the striatum (Ikemoto 2007). Medial VTA DA
neurons (IF, RLI, medial PN)) project to the medial part of the
nucleus accumbens shell (mAcbSh) as well as the medial part of the
olfactory tubercle (OT) while lateral VTA DA neurons (PBP, lateral PN,
PIF) project to the ventral part of the nucleus accumbens shell
(AcbSh). In accordance with a higher level of DA cell loss in the
lateral than medial VTA, TH-positive projections in the ventral part
of the AcbSh were substantially fewer on the lesioned side while more
preserved in the medial part of the mAcbSh as well as the medial part
of the OT (Figure 2B).
TH-positive projections and fibres were preserved in the lesioned
hemisphere in structures that are known to be unaffected by the
injected 6-OHDA toxin (Supplementary figure 1). This included areas
known for receiving dopaminergic input either from other regions than
the SNc and VTA, or from noradrenergic (NA) areas (Canteras et al.
1992; Hasue and Shammah-Lagnado 2002; Matthews et al. 2016), including
the dorsal part of the bed nucleus of the stria terminalis (BNST), the
central nucleus of the amygdala (CeA), and the posterior basolateral
nucleus of the amygdala (BLP) (Supplementary figure 1). In accordance
with the preservation of these TH-positive fibres, TH-positive cell
bodies were found in the paraventricular part of the posterior
hypothalamus (PH), the periaqueductal grey matter (PAG), the dorsal
raphe nucleus (DRN) and the locus coeruleus (LC) (Supplementary figure 1).
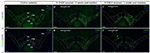
The decrease of TH protein upon 6-OHDA lesioning was confirmed by
fluorescent in situ hybridization (FISH) experiments
validating the presence of Th mRNA (Figure 3). In addition to Th,
Vmat2 mRNA, encoding the Vesicular monoamine transporter (VMAT2) was
assessed.
In the non-lesioned side of 6-OHDA-treated mice (Figure 3,
Supplementary figure 2), and in control mice (vehicle-treated; Figure
3, Supplementary figure 2), both Th and Vmat2 mRNA were present
throughout the SNc and VTA. This is in accordance with the literature
(Adelbrecht et al., 1996; Morales and Margolis, 2017). On the lesioned
side, however, a strong reduction of both Th and Vmat2 mRNAs was
observed. This was most prominent in the SNc, where no or only few,
Th-positive or Vmat2-positive neurons could be observed, thus further
validating the success of our modified protocol for the 6-OHDA lesion
model. When comparing mice sacrificed two and three weeks
post-injection, the same amount of labeling was observed for both Th
and Vmat2 mRNAs (Figure 3A’-A’’,
3B’-B’’). In both these cohorts of mice, no or very
little labeling for Th or Vmat2 could be observed in the SNc on the
lesioned side while the non-lesioned side remained intact. Thus, there
is no difference in detection levels for Th or Vmat2 mRNAs after two
weeks post-injection and when mice have been kept alive for three
weeks after the injection (Figure 3A-B for controls,
3A’-B’ for two weeks post-injection and
3A’’-B’’ for three weeks post-injection).
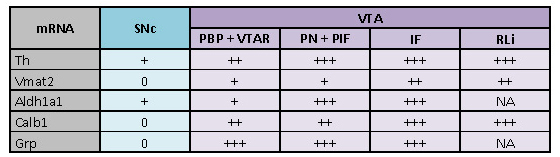
Calb1, Grp and Aldh1a1 mRNA detection levels were decreased in the
SNc and VTA in the lesioned side both two and three weeks after
6-OHDA injection.
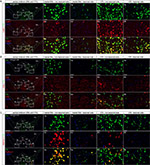
Next, we used three different molecular markers for subtypes of SNc
and VTA DA neurons: Calbindin 1 (Calb1), Gastrin-releasing peptide
(Grp) and Aldehyde dehydrogenase 1 (Aldh1a1). Aldh1a1 has been
described as primarily detected in DA neurons of the medial SNc and
the immediately adjoining ventral aspect of the VTA; Calb1 is
commonly used to define a subtype of DA neurons primarily located in
the VTA, but also present in the SNc; Grp has been reported in a
spatially restricted subtype of VTA DA neurons and rare SNc neurons
(Chung et al. 2005; Greene et al. 2005; Poulin et al. 2018, 2014;
Serra et al. 2021; Viereckel et al. 2016).
First, we verified the detection of SNc and VTA DA neuronal subtypes
as described in the literature in control mice and in the non-lesioned
side of 6-OHDA injected mice. Here, Calb1 mRNA was confirmed to be
present in all VTA subareas and to a much lesser extent in the SNc as
previously shown (Poulin et al. 2014; Viereckel et al. 2016) (Figure
4A and Supplementary figure 2). Grp mRNA was found in sparse neurons
spatially confined to primarily the medio-ventral aspect of the VTA,
and in rare SNc neurons in accordance with the literature (Viereckel
et al. 2016). Grp mRNA was strongest in the PN, PIF and IF with weaker
density in the PBP (Figure 4B and Supplementary figure 2). Aldh1a1
mRNA was detected in both the SNc and VTA with a higher density in the
ventral SNc and ventral aspect of the VTA; a lower density was
detected in the dorsal PBP and lateral SNc (Figure 4C and
Supplementary figure 2). Thus, in control mice, the patterns observed with these molecular
markers were the same as described in the literature (Poulin et al.
2018, 2014; Viereckel et al. 2016).
In contrast, all three molecular markers of VTA and SNc DA neuronal
subtypes were strongly decreased in the 6-OHDA lesioned mice,
primarily in the SNc (Figure 4A-C;
Supplementary figure 2). Quantification of the amount of labeled neurons in the lesioned
compared to non-lesioned side is presented in Table 2.
The duration of the protocol (two or three weeks) did not visibly
affect the severity of the lesion as detected by these markers.
Instead, in both the two-week group and three-week group, the mRNA
detection levels of the three markers were similar. Calb1 mRNA was
nearly absent in the SNc and strongly decreased in the VTA of the
lesioned hemisphere, particularly in the lateral parts of the PBP and
ventral parts of the PN and PIF (Figure 4A and Supplementary figure
2D-D’’, 2G-G’’). Grp mRNA was somewhat
decreased in PBP and ventro-lateral parts of the PN and PIF and lost
in SNc (Figure 4B and Supplementary figure 2E-E’’,
2H-H’’). Aldh1a1 mRNA was decreased in both SNc and VTA in
the lesioned hemisphere, however, some Aldh1a1-positive cells could be
detected (Figure 4C and
Supplementary figure 2F-F’’, 4I-I’’). Taken together with the results of Th and Vmat2
above, these findings verify a substantial decrease of all markers
analyzed for DA neurons in the SNc, with prominent impact also on VTA
DA neurons, in the presented 6-OHDA lesion protocol.
|
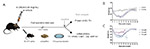
Figure 1: Experimental protocol to induce parkinsonian state
in wild-type mice.
(A) 6-OHDA or vehicle was injected into the median forebrain
bundle (MFB), after which post-operative care included daily
subcutaneous injection of warm saline, access to pellets dipped
in a 15% sucrose solution, and access to a petri dish filled
with 15% sucrose solution placed inside the home cage. Mice were
sacrificed two or three weeks after the 6-OHDA injection, and
brains extracted. Immunohistochemistry and
in situ hybridization were performed to detect
dopaminergic markers in brain sections covering the midbrain
dopamine system. (B) Weight progress of all 6-OHDA lesioned mice
and controls mice (N=15 from day 0 to 14; N=8 from day 15 to
21). (C) Percentage of weight for the three experimental 6-OHDA
groups: Group#0 high dose toxin (2.8 and 3.0 mg/mL 6-OHDA), and
Groups #1 and #2, low dose toxin (1.85 mg/mL 6-OHDA). IHC:
immunohistochemistry; FISH: fluorescent
in situ hybridization. Click image to enlarge |
|
Figure 2: Decrease of TH immunoreactivity in the SNc, VTA, nigrostriatal pathway and striatal complex. (A) Schematique representation of a sagittal section outlining the SNc, VTA, and pathway to the striatum via the median forebrain bundle (MFB) (orange in left picture) with the 6-OHDA (or vehicle) injection in the MFB (left); Coronal representation of SNc and VTA structures, targets of the 6-OHDA injection, outlined in orange (right). (B) Representative immunohistochemistry visualizing dopamine neurons using anti-TH antibody on coronal slices at the midbrain (SNc and VTA) and striatum levels in a 6-OHDA lesioned mouse. VTA subnuclei specified: RLi, IF, PBP, PN, PIF. Striala structures specified: dStr, AcbC, AcbSh, mAcbSh, OT. 6-OHDA lesioned side to the left in the pictures. 6-OHDA: 6-hydroxydopamine; TH: tyrosine hydroxylase; lSNc: lateral substantia nigra pars compacta; SNc: substantia nigra pars compacta; SNr: substantia nigra pars reticulata; PBP: parabrachial pigmented nucleus; PIF: parainterfascicular nucleus; PN: paranigral nucleus; IF: interfascicular nucleus; RLi: rostral linear nucleus; dStr: dorsal striatum; AcbC: nucleus accumbens core; mAcbSh: medial part of the nucleus accumbens shell; OT: olfactory tubercle.
Click image to enlarge |
|
Figure 3: Decrease of Th and Vmat2 mRNAs in both SNc and VTA
in the 6-OHDA-lesioned hemisphere. VTA subnuclei specified: RLi, IF, PBP, PN, PIF. 6-OHDA lesioned
side to the left in the pictures. DAPI used for nuclear
staining. (A and B) Detection of Th mRNA (A) and Vmat2 mRNA (B)
in the SNc and VTA in control mice. (A’ and B’)
Detection of Th mRNA (A’) and Vmat2 mRNA (B’) in the
SNc and VTA in 6-OHDA injected mice, 2 weeks post-lesion.
(A’’ and B’’) Detection of Th mRNA
(A’’) and Vmat2 mRNA (B’’) in the SNc
and VTA in 6-OHDA injected mice, 3 weeks post-lesion. Scale bar:
500 µm. Th: Tyrosine hydroxylase; Vmat2: vesicular monoamine
transporter 2; SNc: substantia nigra pars compacta;
PBP: parabrachial pigmented nucleus; PIF: parainterfascicular
nucleus; PN: paranigral nucleus; IF: interfascicular nucleus;
RLi: rostral linear nucleus. Click image to enlarge |
|
Figure 4: Decrease of DA cell markers Calb1, Aldh1a1, Grp mRNAs in the SNc and VTA in the lesioned hemisphere. 6-OHDA lesioned side to the left in the pictures. Co-localization with Th mRNA (green) for all mRNAs (red) for visualization of dopamine cell bodies. Yellow shows co-localization Th mRNA with the selected mRNA. DAPI (blue) used for nuclear staining. (A) Calb1 mRNA (red) detection in the SNc and VTA in control and 6-OHDA lesioned mice. (B) Grp mRNA (red) detection in the SNc and VTA in control and 6-OHDA lesioned mice. (C) Aldh1a1 mRNA (red) detection in the SNc and VTA in control and 6-OHDA lesioned mice. Th: tyrosine hydroxylase; PBP: parabrachial pigmented nucleus; VTA: ventral tegmental area; PN: paranigral nucleus; PIF: parainterfascicular nucleus; IF: interfascicular nucleus; RLi: rostral linear nucleus ; SNc: substantia nigra pars compacta. All 6-OHDA lesioned data presented here are from the 3 weeks post-injection group. Scale: whole midbrain images: 500µm, close-ups: 50 µm.
Click image to enlarge |
Table 2: Quantitative analysis of SNc and VTA dopamine cell markers in 6-OHDA-treated mice. The table represents the quantified amount of neurons that are left in the lesioned side compared to the non-lesioned, intact, side, as assessed by manual counting. (0) No neurons or very few neurons left; (+) More than a few neurons, up to 30% compared to intact side; (++) 30-50% of the amount on the intact side; (+++) 50-80% of the amount on the intact side; (++++) More than 80% of the amount in the intact side; (NA) Not applicable, applies to areas where the specified mRNA is lacking (in accordance with previous literature). PBP: parabrachial pigmented nucleus; VTAR: ventral tegmental area, rostral part; PN: paranigral nucleus ; PIF: parainterfascicular nucleus; IF: interfascicular nucleus; RLi: rostral linear nucleus ; SNc: substantia nigra pars compacta.
Discussion
In this study, we investigated how well-being and survival of mice treated with the 6-OHDA toxin to generate parkinsonian symptoms could be improved. This is important as data reliability is severely reduced if mice suffer, or even die, during experimental procedures. Even more importantly, from ethical and moral standpoints, experimental protocols that reduce suffering of laboratory animals are needed. In this study, the Refine and Reduce parameters of the 3Rs principle in experimental animal welfare (Tannenbaum and Bennett 2015) were specifically addressed. By improving well-being and survival, the presented protocol will allow researchers to maintain a high quality of life for mice in the 6-OHDA lesion model while also securing reliable data so that fewer mice can be used and unnecessary deaths avoided.
We tested changing three critical experimental parameters in order to
improve well-being and survival of mice while also improving the
experimental protocol and its reproducibility for researchers. Based
on current literature, we introduced changes that would affect several
phases of the experimental procedure. The changes were: 1) reduced
dose of the 6-OHDA toxin; 2) careful post-surgery care (nutrition and
warmth); 3) reduced time until sacrifice. The results clearly show
that mice coped well when administered a lower dose than commonly used
despite displaying the characteristic signs (rotations) of
6-OHDA-induced motor impairment. A high survival rate was observed,
while effectiveness of the toxin could be confirmed by histological
analysis upon sacrifice, both two and three weeks post-lesion. Using a
range of molecular markers, including some of the recently described
markers for subtypes of dopamine neurons, the protocol was carefully
validated.
In accordance with the Reduce parameter, a limited number of mice were
used for the study while maintaining the number necessary to produce
results sufficient to draw relevant conclusions. 22 mice were used for
the lesion protocol including motor assessment; 10 of these mice were
investigated histologically. A step-by-step protocol detailing the
precautions taken is provided here to allow any researcher to follow
the same procedure. We believe that this protocol should prove
beneficial to researchers wanting to study various aspects of PD using
mice, not least in the combination with viral-genetics methodology
such as optogenetics, chemogenics and various fluorescent probes. The
protocol was developed using wild-type C57BL/6NTac mice, but it should
be applicable to other mouse strains following testing and validation.
The 6-OHDA parkinsonian model is long-established to induce strong
impairments in locomotion. However, due to toxin-induced difficulties
in feeding and hydration, current literature clearly shows that
animals commonly fail to thrive and that the survival rate can be
disturbingly low (Table 1). To improve well-being and survival,
addressing the Refine parameter is crucial. By improving
post-operative care, as indicated by recent literature (Boix et al.
2015; Francardo et al. 2011; Rentsch et al. 2019), to include
prevention of hypothermia by subcutaneous injection of pre-heated
saline solution, and by providing nutrition in different forms that
could readily be obtained by the lesioned mice while in recovery, most
mice increased strongly in weight after surgery and appeared healthy
upon inspection. Taken together, these precautions, in combination
with the low dose of the toxin, likley contributed to the well-being
and high level of survival. The 6-OHDA concentration used in this
study was 1.85 mg/ml free-base while the usual concentration reported
in the literature varies between 2.5 to 3.6 mg/ml free-base (Bagga et
al. 2015; Francardo et al. 2011; Sanders and Jaeger 2016). Comparison
between results obtained two and three weeks post-surgery confirmed
the full expression of the lesion phenotype after two weeks. Thus, it
is unnecessary to leave mice lesioned for three weeks when the
phenotype has reached completion already after two weeks. This
shortening of experimental time post-lesion should be an advantage if
an experiment includes subsequent post-mortem analyses, but also for
any experiments that require additional procedures, such as
combination with other types of surgery and/or functional recordings
or assessments. Furthermore, the two-week protocol should be
sufficient for a complete study of down-stream events following DA
cell death in this optimized mouse model of PD.
The 6-OHDA lesion caused a strong decrease of TH-IR in the DA cell
bodies of the SNc and partially in the VTA, particularly the lateral
VTA subnuclei. These results confirmed previous results showing that
6-OHDA injected in the MFB preferentially destroys DA neurons of the
SNc compared to VTA DA neurons (Heuer et al. 2012; Tan et al. 2000).
The results were confirmed by fluorescent
in situ hybridization analyzing the presence of Th, Vmat2,
Calb1, Grp and Aldh1a1 mRNA, known markers of DA metabolism (Th,
Vmat2) as well as subpopulations/subtypes of distinct DA neurons in
the VTA and SNc (Calb1, Grp, Aldh1a1) (Chung et al. 2005; Poulin et
al. 2014; Viereckel et al. 2016; Serra et al 2021). Indeed, the
presence and distribution of all mRNAs analyzed were decreased
uniquely on the lesioned side in the SNc and ventro-lateral VTA,
demonstrating that this loss of mRNA labeling was due to neuronal
degeneration following the 6-OHDA injection. In accordance with the
loss of TH-IR in the ventral midbrain (SNc and VTA), TH-positive
fibres were nearly absent from the dorsal and ventral striatum in the
lesioned hemisphere, with the exception of the medial mAcbSh. However,
the maintenance of TH fibres in this area is in accordance with the
preservation of DA neurons in the medial VTA, including the IF and
medial PN, which project to the mAcbSh (Ikemoto 2007). The decrease of
TH observed with the low concentration of 6-OHDA is in accordance with
a previous study showing partial loss of TH-IR in mice injected with a
0.7 mg/ml 6-OHDA solution, analysed five weeks post-lesion (Boix et
al. 2015). Another study showed a decrease of TH-IR in the striatum
one week post-lesion but used a higher concentration of the 6-OHDA
toxin (15 mg/mL) (Rentsch et al. 2019). Here, we combined a lower dose
of the 6-OHDA toxin with a short interval between injection and
sacrifice to optimize the protocol in respect of both well-being and
survival, and hence, reliability of the data.
Although the 6-OHDA model mimics many parkinsonian features, it also
has limitations. Firstly, it does not induce the formation of Lewy
bodies, an important hallmark of PD. Secondly, the 6-OHDA neurotoxin
does not pass the blood brain barrier and has to be injected
intracerebrally into the dorsal striatum, the MFB or the SNc to induce
degeneration of the nigrostriatal pathway and the associated PD-like
symptoms. Thirdly, the 6-OHDA model is acute and does not cause a slow
progression of DA neuron degeneration. These limitations are important
to bear in mind when interpreting results from this model. However,
while not representing the full repertoire of PD pathology observed in
humans, the potent degeneration of SNc and VTA DA neurons in the
6-OHDA model makes it attractive for experimental studies centered
around the critical aspect of DA cell degeneration in PD. A
mouse-based protocol with improved health status for the experimental
animals despite substantial DA cell death is therefore important to
achieve.
In general, only a limited number of studies using the 6-OHDA model in
mice have reported the survival rate of the animals. This makes it
difficult for other researchers to assess the severity and
replicability of the procedure on the animals in each study. However,
upon scrutinizing current literature, we extracted and summarized the
descriptions provided in Methods and Results sections of published
reports (Table 1). Several studies that have used the 6-OHDA lesion
model in mice or rats were addressed. However, due to lack of
information provided in many studies using a 6-OHDA model in rodents,
the table may not be complete. Despite the difficulty in comparing
studies that have used different conditions (species, injections
sites, volume, dose, and various types of post-operative care), we can
conclude that the use of post-operative care is essential to reach a
high survival rate of mice (80 to 100%). In the present study, we
could demonstrate a survival rate of 86.4-90.9% by reducing the
concentration of 6-OHDA and implementing post-operative care. Our
findings are in accordance with other recent studies that also have
aimed to improve survival of mice undergoing the 6-OHDA lesion
procedure (Bagga et al. 2015; Boix et al. 2015; Francardo et al. 2011;
Grealish et al. 2010; Heuer et al. 2012; Koski et al. 2019; Lundblad
et al. 2004; Masini et al. 2021; Rentsch et al. 2019; Sanders and
Jaeger 2016; Thiele et al., 2012). The post-operative care employed in
our study was mainly based on a literature search performed in 2016,
which included the following references: Bagga et al. 2015; Francardo
et al. 2011; Grealish et al. 2010; Heuer et al. 2012; Sanders and
Jaeger 2016; Thiele et al. 2012. However, more recently , other
studies have demonstrated improved protocols that have included
additional pre- and post-operative care of the mice (Koski et al.
2019; Masini et al. 2021; Rentsch et al. 2019). These protocols
included handling of animals prior to surgery to reduce stress (Koski
et al. 2019; Masini et al. 2021) and hand-feeding of animals after
surgery to certify their feeding (Boix et al. 2015; Francardo et al.
2011; Koski et al. 2019). Implementing such precautions might indeed
increase survival rate even further, and should be considered for
future applications of the 6-OHDA model.
A possible limitation of the present study is the low number of mice
used (10 mice for the full procedure including histological analysis;
22 mice in total for assessment of survival rate). However, this
limitation is consistent with the animal welfare perspective (3Rs) of
reducing the number of animals sacrificed for research purposes, while
still obtaining relevant and useful results. Importantly, survival
rate indeed increased by using the current protocol. Furthermore, by
using a number of different established markers for the DA neuron
population, including more recently described markers for subtypes of
DA neurons, to maximise the output derived from these experimental
mice, the study has allowed detailed histological examination
throughout each lesioned mouse brain. Detailed information of the
extent of dopaminergic markers in both the SNc and each VTA subnucleus
upon implementation of the current 6-OHDA-lesion protocol is thereby
available.
In summary, mice coped well throughout the current 6-OHDA lesion
protocol, a high survival rate was observed, and the toxin-induced
phenotype was confirmed by spontaneous rotations as well as by loss of
a range of molecular markers of the midbrain DA system. The current
protocol could allow future studies to implement the 6-OHDA mouse
model with an improved perpective in terms of animal welfare, and in
combination with advanced experimental procedures could help to
identify and validate treatment methods for PD.
Conclusions
By here improving the 6-OHDA model in mice, the 3Rs principle (Replace, Refine, Reduce) in experimental animal welfare can be followed more closely, and the Refine and Reduce parameters fulfilled even in advanced experiments aiming to enhance current knowledge and treat complex human disorders.
Ethics approval
The animal study was reviewed and approved by Uppsala Ethical
Committee for Laboratory Animal Research (Uppsala djurförsöksetiska
nämnd, Uppsala Tingsrätt, Box 1113, 751 41 Uppsala).
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article and its additional files. Correspondence and request for additionnal data or material should be addressed to the corresponding author.
Conflict of interest
The author declared no conflict of interest.
Conflict of interest
This work was funded by Uppsala University and by grants to Å.W.M from the Swedish Research Council (SMRC 2017-02039), the Swedish Brain Foundation (Hjärnfonden), Parkinsonfonden, and the Research Foundations of Bertil Hållsten, Zoologiska stiftelsen, and Åhlén.
Authors’ contributions
AG: Investigation, formal analysis, methodology, literature review table summary, writing - original draft and review; BV: Investigation, formal analysis, literature review table summary; ÅM: Conceptualization, formal analysis, funding acquisition, project administration, supervision, writing - review and editing.
Acknowledgments
We thank Dr Sylvie Dumas (Oramacell) for providing primers, probes and protocol for the in situ hybridization experiments, and Uppsala University rodent veterinarians and animal facility staff for helpful collaboration. We apologize to any authors of studies that we might have overlooked in our summary of current literature on the 6-OHDA model in mice. Based on the results of the literature search, we encourage authors to declare state of well-being and survival rate when implementing a 6-OHDA-lesion protocol to enable enhanced stringency and transparency in these kinds of studies.
References
-
Accolla, E.A., Pollo, C., (2019). Mood effects after deep brain stimulation for Parkinson’s disease: An update. Fronties in Neurology. 10, 617. https://doi.org/10.3389/fneur.2019.00617
- Adelbrecht, C., Agid, Y., Raisman-Vozari, R., (1996). Effect of the weaver mutation on the expression of dopamine membrane transporter, tyrosine hydroxylase and vesicular monoamine transporter in dopaminergic neurons of the substantia nigra and the ventral tegmental area. Molecular Brain Research. 43, 291-300. https://doi.org/10.1016/S0169-328X(96)00214-8
- Alexander, G.E., Crutcher, M.D., DeLong, M.R., (1990). Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Progress in Brain Research. 85, 119-146.
- Armstrong, M.J., Okun, M.S., (2020). Diagnosis and treatment of Parkinson disease: A review. The Journal of the American Medical Association. 323, 548. https://doi.org/10.1001/jama.2019.22360
- Bagga, V., Dunnett, S.B., Fricker, R.A., (2015). The 6-OHDA mouse model of Parkinson’s disease – Terminal striatal lesions provide a superior measure of neuronal loss and replacement than median forebrain bundle lesions. Behavioural Brain Research. 288, 107–117. https://doi.org/10.1016/j.bbr.2015.03.058
- Barker, R.A., Williams-Gray, C.H., (2016). Review: The spectrum of clinical features seen with alpha synuclein pathology. Neuropathology and Applied Neurobiology. 42, 6-19. https://doi.org/10.1111/nan.12303
- Benabid, A.L., Pollak, P., Gross, C., Hoffmann, D., Benazzouz, A., Gao, D.M., Laurent, A., Gentil, M., Perret, J., (1994). Acute and long-term effects of subthalamic nucleus stimulation in Parkinson’s disease. Stereotactic and Functional Neurosurgery. 62, 76–84. https://doi.org/10.1159/000098600
- Bichler, E.K., Cavarretta, F., Jaeger, D., (2021). Changes in excitability properties of ventromedial motor thalamic neurons in 6-OHDA lesioned mice. eNeuro. 8. https://doi.org/10.1523/ENEURO.0436-20.2021
- Björklund, A., Dunnett, S.B., (2007). Dopamine neuron systems in the brain: an update. Trends in Neurosciences. 30, 194-202. https://doi.org/10.1016/j.tins.2007.03.006
- Blum, D., Torch, S., Lambeng, N., Nissou, M.-F., Benabid, A.-L., Sadoul, R., Verna, J.-M., (2001). Molecular pathways involved in the neurotoxicity of 6-OHDA, dopamine and MPTP: contribution to the apoptotic theory in Parkinson’s disease. Progress in Neurobiology.65, 135-172. https://doi.org/10.1016/S0301-0082(01)00003-X
- Boix, J., Padel, T., Paul, G., (2015). A partial lesion model of Parkinson’s disease in mice – Characterization of a 6-OHDA-induced medial forebrain bundle lesion. Behavioural Brain Research. 284, 196-206. https://doi.org/10.1016/j.bbr.2015.01.053
- Bonito-Oliva, A., Masini, D., Fisone, G., (2014). A mouse model of non-motor symptoms in Parkinson’s disease: focus on pharmacological interventions targeting affective dysfunctions. Frontiers in Behavioral Neuroscience. 8. https://doi.org/10.3389/fnbeh.2014.00290
- Braak, H., Tredici, K.D., Rüb, U., de Vos, R.A.I., Jansen Steur, E.N.H., Braak, E., (2003). Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiology of Aging. 24, 197-211. https://doi.org/10.1016/S0197-4580(02)00065-9
- Canteras, N.S., Simerly, R.B., Swanson, L.W., (1992). Connections of the posterior nucleus of the amygdala. Journal of Comparative Neurology. 324, 143-179. https://doi.org/10.1002/cne.903240203
- Cenci, M.A., Björklund, A., (2020). Animal models for preclinical Parkinson’s research: An update and critical appraisal. In: Progress in Brain Research. Elsevier, pp. 27-59. https://doi.org/10.1016/bs.pbr.2020.02.003
- Chaudhuri, K.R., Healy, D.G., Schapira, A.H., (2006). Non-motor symptoms of Parkinson’s disease: diagnosis and management. The Lancet Neurology. 5, 235-245. https://doi.org/10.1016/S1474-4422(06)70373-8
- Chiken, S., Nambu, A., (2016). Mechanism of deep brain stimulation: inhibition, excitation, or disruption? Neuroscientist. 22, 313-322. https://doi.org/10.1177/1073858415581986
- Chung, C.Y., Seo, H., Sonntag, K.C., Brooks, A., Lin, L., Isacson, O., (2005). Cell type-specific gene expression of midbrain dopaminergic neurons reveals molecules involved in their vulnerability and protection. Human Molecular Genetics.14, 1709-1725. https://doi.org/10.1093/hmg/ddi178
- Connolly, B.S., Lang, A.E., (2014). Pharmacological treatment of Parkinson disease: A review. The Journal of the American Medical Association. 311, 1670. https://doi.org/10.1001/jama.2014.3654
- Cyranoski, D., (2018). ‘Reprogrammed’ stem cells implanted into patient with Parkinson’s disease. Nature.d41586-018-07407-9. https://doi.org/10.1038/d41586-018-07407-9
- Dawson, T.M., Ko, H.S., Dawson, V.L., (2010). Genetic animal models of Parkinson’s disease. Neuron. 66, 646-661. https://doi.org/10.1016/j.neuron.2010.04.034
- Dumas, S., Wallén-Mackenzie, Å., (2019). Developmental co-expression of Vglut2 and Nurr1 in a Mes-Di-encephalic continuum preceeds dopamine and glutamate neuron specification. Frontiers in Cell and Developmental Biology. 7, 307. https://doi.org/10.3389/fcell.2019.00307
- Eisinger, R.S., Cernera, S., Gittis, A., Gunduz, A., Okun, M.S., (2019). A review of basal ganglia circuits and physiology: Application to deep brain stimulation. Parkinsonism & Related Disorders. 59, 9-20. https://doi.org/10.1016/j.parkreldis.2019.01.009
- European Union, (2010). Directive 63/2010 / EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. Official Journal of the European Union L 276 of 20/10/2010.
- Felgenhauer, J.L., Brune, J.E., Long, M.E., Manicone, A.M., Chang, M.Y., Brabb, T.L., Altemeier, W.A., Frevert, C.W., (2020). Evaluation of nutritional gel supplementation in C57BL/6J mice infected with mouse-adapted influenza A/PR/8/34 virus. Comparative Medicine. 70, 471–486. https://doi.org/10.30802/AALAS-CM-20-990138
- Francardo, V., Recchia, A., Popovic, N., Andersson, D., Nissbrandt, H., Cenci, M.A., (2011). Impact of the lesion procedure on the profiles of motor impairment and molecular responsiveness to L-DOPA in the 6-hydroxydopamine mouse model of Parkinson’s disease. Neurobiology of Disease. 42, 327–340. https://doi.org/10.1016/j.nbd.2011.01.024
- Glinka, Y., Gassen, M., Youdim, M.B.H., (1997). Mechanism of 6-hydroxydopamine neurotoxicity, in: Riederer, P., Calne, D.B., Horowski, R., Mizuno, Y., Poewe, W., Youdim, M.B.H. (Eds.), Advances in research on neurodegeneration, Journal of Neural Transmission. Supplementa. Springer Vienna, Vienna, pp. 55-66. https://doi.org/10.1007/978-3-7091-6842-4_7
- Graybiel, A.M., (2000). The basal ganglia. Current Biology. 10, R509–R511. https://doi.org/10.1016/S0960-9822(00)00593-5
- Grealish, S., Mattsson, B., Draxler, P., Björklund, A., (2010). Characterisation of behavioural and neurodegenerative changes induced by intranigral 6-hydroxydopamine lesions in a mouse model of Parkinson’s disease. European Journal of Neuroscience. 31, 2266-2278. https://doi.org/10.1111/j.1460-9568.2010.07265.x
- Grealish, S., Diguet, E., Kirkeby, A., Mattsson, B., Heuer, A., Bramoulle, Y., Van Camp, N., Perrier, A.L., Hantraye, P., Björklund, A., Parmar, M., (2014). Human ESC-derived dopamine neurons show similar preclinical efficacy and potency to fetal neurons when grafted in a rat model of Parkinson’s disease. Cell Stem Cell 15, 653–665. https://doi.org/10.1016/j.stem.2014.09.017
- Greene, J.G., Dingledine, R., Greenamyre, J.T., (2005). Gene expression profiling of rat midbrain dopamine neurons: implications for selective vulnerability in parkinsonism. Neurobiology of Disease. 18, 19-31. https://doi.org/10.1016/j.nbd.2004.10.003
- Hasue, R.H., Shammah-Lagnado, S.J., (2002). Origin of the dopaminergic innervation of the central extended amygdala and accumbens shell: A combined retrograde tracing and immunohistochemical study in the rat. Journal of Comparative Neurology. 454, 15-33. https://doi.org/10.1002/cne.10420
- Hernandez-Baltazar, D., Zavala-Flores, L.M., Villanueva-Olivo, A., (2017). The 6-hydroxydopamine model and parkinsonian pathophysiology: Novel findings in an older model. Neurología (English Edition). 32, 533-539. https://doi.org/10.1016/j.nrleng.2015.06.019
- Herrington, T.M., Cheng, J.J., Eskandar, E.N., (2016). Mechanisms of deep brain stimulation. Journal of Neurophysiology. 115, 19-38. https://doi.org/10.1152/jn.00281.2015
- Heuer, A., Smith, G.A., Lelos, M.J., Lane, E.L., Dunnett, S.B., (2012). Unilateral nigrostriatal 6-hydroxydopamine lesions in mice I: Motor impairments identify extent of dopamine depletion at three different lesion sites. Behavioural Brain Research. 228, 30-43. https://doi.org/10.1016/j.bbr.2011.11.027
- Heywood, P., Gill, S., (1997). Bilateral dorsolateral subthalamotomy for advanced Parkinson’s disease. The Lancet.350, 1224. https://doi.org/10.1016/S0140-6736(05)63455-1
- Ikemoto, S., (2007). Dopamine reward circuitry: Two projection systems from the ventral midbrain to the nucleus accumbens–olfactory tubercle complex. Brain Research Reviews. 56, 27-78. https://doi.org/10.1016/j.brainresrev.2007.05.004
-
Im, H.-J., Hahm, J., Kang, H., Choi, H., Lee, H., Hwang, D.W., Kim,
E.E., Chung, J.-K., Lee, D.S., (2016). Disrupted brain metabolic
connectivity in a 6-OHDA-induced mouse model of Parkinson’s
disease examined using persistent homology-based analysis.
Scientific Reports. 6, 33875.
https://doi.org/10.1038/srep33875
Kalia, L.V., Lang, A.E., (2015). Parkinson’s disease. The Lancet. 386, 896-912. https://doi.org/10.1016/S0140-6736(14)61393-3' Khan, A.U., Akram, M., Daniyal, M., Zainab, R., (2019). Awareness and current knowledge of Parkinson’s disease: a neurodegenerative disorder. International Journal of Neuroscience. 129, 55-93. https://doi.org/10.1080/00207454.2018.1486837 -
Kim, H.-J., Jeon, B.S., Paek, S.H., (2015). Nonmotor symptoms and
subthalamic deep brain stimulation in Parkinson’s disease.
The Journal of Molecular Diagnostics.8, 83-91.
https://doi.org/10.14802/jmd.15010
Kin, K., Yasuhara, T., Kameda, M., Date, I., (2019). Animal models for Parkinson’s disease research: Trends in the 2000s. International Journal of Molecular Sciences. 20, 5402. https://doi.org/10.3390/ijms20215402 - Kordower, J.H., Olanow, C.W., Dodiya, H.B., Chu, Y., Beach, T.G., Adler, C.H., Halliday, G.M., Bartus, R.T., (2013). Disease duration and the integrity of the nigrostriatal system in Parkinson’s disease. Brain. 136, 2419-2431. https://doi.org/10.1093/brain/awt192
- Koski, S.K., Leino, S., Rannanpää, S., Salminen, O., (2019). Implementation of improved postoperative care decreases the mortality rate of operated mice after an abundant 6-hydroxydopamine lesion of nigrostriatal dopaminergic neurons. Scandinavian Journal of Laboratory Animal Sciences. 45, 1-11. https://doi.org/10.23675/SJLAS.V45I0.581
- Lecker, J., Froberg-Fejko, K., (2016). Using environmental enrichment and nutritional supplementatioto improve breeding success in rodents. Laboratory Animals. 45, 406-407. https://doi.org/10.1038/laban.1114
- LeWitt, P.A., (2015). Levodopa therapy for Parkinson’s disease: Pharmacokinetics and pharmacodynamics. Movement Disorders. 30, 64-72. https://doi.org/10.1002/mds.26082
- Lorenc-Koci, E., Wolfarth, S., Ossowska, K., (1996). Haloperidol-increased muscle tone in rats as a model of parkinsonian rigidity. Experimental Brain Research.109. https://doi.org/10.1007/BF00231786
- Lundblad, M., Picconi, B., Lindgren, H., Cenci, M.A., (2004). A model of l-DOPA-induced dyskinesia in 6-hydroxydopamine lesioned mice: relation to motor and cellular parameters of nigrostriatal function. Neurobiology of Disease. 16, 110-123. https://doi.org/10.1016/j.nbd.2004.01.007
- Masini, D., Plewnia, C., Bertho, M., Scalbert, N., Caggiano, V., Fisone, G., (2021). A Guide to the Generation of a 6-Hydroxydopamine Mouse Model of Parkinson’s Disease for the Study of Non-Motor Symptoms. Biomedicines. 9, 598. https://doi.org/10.3390/biomedicines9060598
- Matthews, G.A., Nieh, E.H., Vander Weele, C.M., Halbert, S.A., Pradhan, R.V., Yosafat, A.S., Glober, G.F., Izadmehr, E.M., Thomas, R.E., Lacy, G.D., Wildes, C.P., Ungless, M.A., Tye, K.M., (2016). Dorsal raphe dopamine neurons represent the experience of social isolation. Cell. 164, 617-631. https://doi.org/10.1016/j.cell.2015.12.040
- Miocinovic, S., Somayajula, S., Chitnis, S., Vitek, J.L., (2013). History, applications, and mechanisms of deep brain stimulation. The Journal of the American Medical Association - Neurology. 70, 163. https://doi.org/10.1001/2013.jamaneurol.45
- Morales, M., Margolis, E.B., (2017). Ventral tegmental area: cellular heterogeneity, connectivity and behaviour. Nature Reviews Neuroscience. 18, 73–85. https://doi.org/10.1038/nrn.2016.165
-
Muñoz, A., Lopez-Lopez, A., Labandeira, C.M., Labandeira-Garcia,
J.L., (2020). Interactions between the serotonergic and other
neurotransmitter systems in the basal ganglia: Role in
Parkinson’s disease and adverse effects of L-DOPA.
Frontiers in Neuroanatomy. 14, 26.
https://doi.org/10.3389/fnana.2020.00026
Obeso, J.A., Stamelou, M., Goetz, C.G., Poewe, W., Lang, A.E., Weintraub, D., Burn, D., Halliday, G.M., Bezard, E., Przedborski, S., Lehericy, S., Brooks, D.J., Rothwell, J.C., Hallett, M., DeLong, M.R., Marras, C., Tanner, C.M., Ross, G.W., Langston, J.W., Klein, C., Bonifati, V., Jankovic, J., Lozano, A.M., Deuschl, G., Bergman, H., Tolosa, E., Rodriguez-Violante, M., Fahn, S., Postuma, R.B., Berg, D., Marek, K., Standaert, D.G., Surmeier, D.J., Olanow, C.W., Kordower, J.H., Calabresi, P., Schapira, A.H.V., Stoessl, A.J., (2017). Past, present, and future of Parkinson’s disease: A special essay on the 200th Anniversary of the Shaking Palsy. Movement Disorders. 32, 1264–1310. https://doi.org/10.1002/mds.27115 - Olanow, C.W., Stocchi, F., (2018). Levodopa: A new look at an old friend. Movement Disorders.. 33, 859-866. https://doi.org/10.1002/mds.27216
- Paredes-Rodriguez, E., Vegas-Suarez, S., Morera-Herreras, T., De Deurwaerdere, P., Miguelez, C., (2020). The noradrenergic system in Parkinson’s disease. Frontiers in Pharmacology. 11, 435. https://doi.org/10.3389/fphar.2020.00435
- Park, A., Stacy, M., (2009). Non-motor symptoms in Parkinson’s disease. Journal of Neurology. 256, 293-298. https://doi.org/10.1007/s00415-009-5240-1
- Park, S.E., Song, K.-I., Kim, H., Chung, S., Youn, I., (2018). Graded 6-OHDA-induced dopamine depletion in the nigrostriatal pathway evokes progressive pathological neuronal activities in the subthalamic nucleus of a hemi-parkinsonian mouse. Behavioural Brain Research. 344, 42–47. https://doi.org/10.1016/j.bbr.2018.02.014
- Parmar, M., Grealish, S., Henchcliffe, C., (2020). The future of stem cell therapies for Parkinson disease. Nature Reviews Neuroscience. 21, 103-115. https://doi.org/10.1038/s41583-019-0257-7'
- Percie du Sert, N., Ahluwalia, A., Alam, S., Avey, M.T., Baker, M., Browne, W.J., Clark, A., Cuthill, I.C., Dirnagl, U., Emerson, M., Garner, P., Holgate, S.T., Howells, D.W., Hurst, V., Karp, N.A., Lazic, S.E., Lidster, K., MacCallum, C.J., Macleod, M., Pearl, E.J., Petersen, O.H., Rawle, F., Reynolds, P., Rooney, K., Sena, E.S., Silberberg, S.D., Steckler, T., Würbel, H., (2020). Reporting animal research: Explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biology. 18, e3000411. https://doi.org/10.1371/journal.pbio.3000411
- Petry-Schmelzer, J.N., Krause, M., Dembek, T.A., Horn, A., Evans, J., Ashkan, K., Rizos, A., Silverdale, M., Schumacher, W., Sack, C., Loehrer, P.A., Fink, G.R., Fonoff, E.T., Martinez-Martin, P., Antonini, A., Barbe, M.T., Visser-Vandewalle, V., Ray-Chaudhuri, K, Timmermann, L., Dafsari, H.S. on behalf of EUROPAR and the IPMDS Non-Motor PD Study Group, (2019). Non-motor outcomes depend on location of neurostimulation in Parkinson’s disease. Brain. 142, 3592-3604. https://doi.org/10.1093/brain/awz285
- Pfeiffer, R.F., (2016). Non-motor symptoms in Parkinson’s disease. Parkinsonism & Related Disorders. 22, S119-S122. https://doi.org/10.1016/j.parkreldis.2015.09.004
- Poulin, J.-F., Zou, J., Drouin-Ouellet, J., Kim, K.-Y.A., Cicchetti, F., Awatramani, R.B., (2014). Defining Midbrain Dopaminergic Neuron Diversity by Single-Cell Gene Expression Profiling. Cell Reports. 9, 930-943. https://doi.org/10.1016/j.celrep.2014.10.008
- Poulin, J.-F., Caronia, G., Hofer, C., Cui, Q., Helm, B., Ramakrishnan, C., Chan, C.S., Dombeck, D.A., Deisseroth, K., Awatramani, R., (2018). Mapping projections of molecularly defined dopamine neuron subtypes using intersectional genetic approaches. Nature Neuroscience. 21, 1260-1271. https://doi.org/10.1038/s41593-018-0203-4
- Rentsch, P., Stayte, S., Morris, G.P., Vissel, B., (2019). Time dependent degeneration of the nigrostriatal tract in mice with 6-OHDA lesioned medial forebrain bundle and the effect of activin A on l-Dopa induced dyskinesia. BMC Neuroscience. 20, 5. https://doi.org/10.1186/s12868-019-0487-7
- Ribeiro, R.P., Moreira, E.L.G., Santos, D.B., Colle, D., dos Santos, A.A., Peres, K.C., Figueiredo, C.P., Farina, M., (2013). Probucol affords neuroprotection in a 6-OHDA mouse model of Parkinson’s disease. Neurochemical Research. 38, 660-668. https://doi.org/10.1007/s11064-012-0965-0
-
Rispoli, V., Schreglmann, S.R., Bhatia, K.P., (2018). Neuroimaging
advances in Parkinson’s disease.
Current Opinion in Neurology. 31, 415-424.
https://doi.org/10.1097/WCO.0000000000000584
Sanders, T.H., Jaeger, D., (2016). Optogenetic stimulation of cortico-subthalamic projections is sufficient to ameliorate bradykinesia in 6-ohda lesioned mice. Neurobiology of Disease. 95, 225-237. https://doi.org/10.1016/j.nbd.2016.07.021 -
Schneider, S.A., Obeso, J.A., (2015). Clinical and pathological
features of Parkinson’s disease In: Nguyen, H.H.P., Cenci,
M.A. (Eds.), Behavioral neurobiology of Huntington’s disease
and Parkinson’s disease. Springer Berlin Heidelberg, Berlin,
Heidelberg, pp. 205-220. https://doi.org/10.1007/7854_2014_317
Senek, M., Nyholm, D., (2014). Continuous drug delivery in Parkinson’s disease. CNS Drugs. 28, 19-27. https://doi.org/10.1007/s40263-013-0127-1 - Serra, G.P., Guillaumin, A., Dumas, S., Vlcek, B., Wallén-Mackenzie, Å., (2021). Midbrain dopamine neurons defined by TrpV1 modulate psychomotor behavior. Frontiers in Neural Circuits. 15, 726893. https://doi.org/10.3389/fncir.2021.726893
- Sesack, S.R., Grace, A.A., (2010). Cortico-basal ganglia reward network: Microcircuitry. Neuropsychopharmacology. 35, 27-47. https://doi.org/10.1038/npp.2009.93
-
Spillantini, M.G., Schmidt, M.L., Lee, V.M.-Y., Trojanowski, J.Q.,
Jakes, R., Goedert, M., (1997). α-Synuclein in Lewy bodies.
Nature. 388, 839-840.
https://doi.org/10.1038/42166
Stayte, S., Rentsch, P., Li, K.M., Vissel, B., (2015). Activin A protects midbrain neurons in the 6-hydroxydopamine mouse model of Parkinson’s disease. PLoS One. 10, e0124325. https://doi.org/10.1371/journal.pone.0124325 - Tan, Y., Williams, E.A., Lancia, A.J., Zahm, D.S., (2000). On the altered expression of tyrosine hydroxylase and calbindin-D 28kD immunoreactivities and viability of neurons in the ventral tegmental area of Tsai following injections of 6-hydroxydopamine in the medial forebrain bundle in the rat. Brain Research. 869, 56-68. https://doi.org/10.1016/S0006-8993(00)02348-9
- Tannenbaum, J., Bennett, B.T., (2015). Russell and Burch’s 3Rs then and now: the need for clarity in definition and purpose. Journal of the American Association for Laboratory Animal Science. 54, 120-132.
- Thiele, S.L., Warre, R., Nash, J.E., 2012. Development of a Unilaterally-lesioned 6-OHDA Mouse Model of Parkinson’s Disease. Journal of Visualized Experiments. 3234. https://doi.org/10.3791/3234
- Tysnes, O.-B., Storstein, A., (2017). Epidemiology of Parkinson’s disease. Journal of Neural Transmission. 124, 901-905. https://doi.org/10.1007/s00702-017-1686-y
- Viereckel, T., Dumas, S., Smith-Anttila, C.J.A., Vlcek, B., Bimpisidis, Z., Lagerström, M.C., Konradsson-Geuken, Å., Wallén-Mackenzie, Å., (2016). Midbrain gene screening identifies a new mesoaccumbal glutamatergic pathway and a marker for dopamine cells neuroprotected in Parkinson’s disease. Scientific Reports. 6, 35203. https://doi.org/10.1038/srep35203
- Yang, K., Zhao, X., Wang, C., Zeng, C., Luo, Y., Sun, T., (2021). Circuit mechanisms of L-DOPA-Induced dyskinesia (LID). Frontiers in Neuroscience. 15, 614412. https://doi.org/10.3389/fnins.2021.614412
- Yuan, H., Sarre, S., Ebinger, G., Michotte, Y., (2005). Histological, behavioural and neurochemical evaluation of medial forebrain bundle and striatal 6-OHDA lesions as rat models of Parkinson’s disease. Journal of Neuroscience Methods. 144, 35-45. https://doi.org/10.1016/j.jneumeth.2004.10.004