Original scientific article
Are female mice dehydrated during peak lactation? Effect of water and gel supplement on hydration parameters and water consumption in two strains of mice
by Charlotta Grims1*, Christina Jacobson1, Patricia Hedenqvist2*
1Centre for Comparative Medicine, Biomedical Centre
(BMC), Lund University, Sweden
2Department of clinical sciences, Swedish University of Agriculture, Uppsala, Sweden k
 Correspondence: ACharlotta Grims, e-mail: charlotta.grims@med.lu.se
Correspondence: ACharlotta Grims, e-mail: charlotta.grims@med.lu.se
Summary
Mice (Mus musculus) have a high basal rate of metabolism which
increases during pregnancy and lactation. During peak lactation, water
intake amounts to up to 65 % of the bodyweight per day. Providing
water in a bottle may pose a restriction of water intake and lead to
dehydration during periods of high demand, such as peak lactation. To
establish if female mice are able to sustain a physiological hydration
status during peak lactation, a completely randomized factorial design
study was conducted with 12 RjOrl:SWISS (SWISS) and 12 C57BL/6JRj (B6)
six-week old female mice in breeding. Female mice were randomly
assigned to one of three groups with different watering alternatives:
water bottle (Standard, n=6); water bottle + sachet with 98 % water
gel (Gel, n=6); or water bottle + water bowl (Bowl, n=6). Non-mated
females, provided with water bottles, served as controls (n=6).
Hydration parameters [total protein (TP), hemoglobin, hematocrit,
serum osmolality (Osmol), blood urea nitrogen (BUN)] and magnesium
were measured in blood before mating (Pre) and during peak lactation
(Peak), and at the same time points in controls. Water bottles were
weighed during lactation and body weights of females and litters
recorded at weaning. Data were analyzed by parametric or
non-parametric methods to evaluate effects of strain, group and time
point. The hydration parameters and magnesium were mostly within
normal ranges in all animals at Pre and Peak. TP was lower at Peak in
all lactating groups compared to Controls and to Pre (p<0.01). Mice
in group Bowl consumed 54 % less bottle water compared with Gel and
Standard (p<0.001), had 34 % lower levels of BUN than Standard and
Control (p<0.01) and 5 % lower serum osmolality at Peak than Pre
(p<0.01).
Conclusion: Female mice are not dehydrated at peak
lactation. However, they prefer to drink, and seemingly drink more
water, from a bowl than from a bottle. concentration are likely to be
the same throughout the cardiovascular system in healthy pigs.
Introduction
Owing to their small body size, mice have a very high rate of mass-specific metabolism. Laboratory mice are generally provided with dry feed, containing approximately 10 % of water, and drinking water in bottles or automated water systems. Adequate water intake is essential for animal welfare, and restriction can reduce performance and increase the risk of diseases. During peak lactation, female mice can increase feed and water intake up to four times, leading to a water intake that amounts to approximately 60 % of their body weight per day (Murai et al. 2013). The watering systems for housing laboratory mice are considered to provide water ad libitum. Water bottles are either equipped with a sipper tube or a cap with a hole from which the water is licked. The volume of each lick depends on the diameter of the orifice and the amount of air in the inverted water bottle, as was shown in rats (Weijnen 1998). In rabbits, drinking water from a nipple drinker has been shown to take longer and result in lower water intake compared with drinking from a bowl (Tschudin et al. 2011). Horses prefer to drink, and drink more, from a bucket compared with an automatic water bowl, even if the automatic water flow is meeting their normal drinking rate (Nyman and Dahlborn 2001).
A pilot study in lactating fancy mice showed that they prefer to drink
from a bowl rather than a bottle. Adding a bowl to the cage increased
water intake by 55 %, whereas bottle water intake decreased by 67 %
(Hedenqvist et al. 2015). The preference for the bowl suggests that
drinking from a bottle is more time consuming. At peak lactation when
much time is spent on the large feed intake and tending to the pups,
drinking from a bottle may pose a restriction for water intake, and
thus be a welfare problem.
To test the hypothesis that lactating mice are dehydrated during peak
lactation, a study was performed in mice of two strains. Water was
provided in bottles, or additionally as gel or in a bowl during
lactation. Parameters of hydration were measured before mating and at
peak lactation, as was the bottle water consumption during lactation.
Water as gel was included as part of the study because it would
provide a more practical means of adding water compared with a bowl.
|
Figure 1. A. The IVC cage with nesting
material, a ladder, and a stainless-steel water bowl attached.
The gel sachet and the water bottle with the perforated cap are
shown next to the cage. B. Close up of the
bottle lid with the 1.2 mm large hole. Click image to enlarge |
Materials & Methods
Animals and housing
RjOrl:SWISS (SWISS) and C57BL/6JRj (B6) mice (12 females and 6 males
per strain) were purchased from Janvier Labs (Le Genest-Saint-Isle,
France) at 6 weeks of age (total n=42). The colony of origin was
monitored according to FELASA recommendations (Mähler et al. 2014) and
declared free from all listed agents. All mice were housed in IVC
cages (Innocage®, Innovive, San Diego, USA) with 54 air changes per
hour. The outside dimensions of the cage were Length: 37.3 x Width:
23.4 x Height: 14.0 cm. Each cage contained corncob bedding, nesting
material (shredded paper) and a ladder for enrichment (Figure 1A). The
cage with enclosed contents was irradiated. Commercial irradiated
pelleted food (SDS RM3, UK) and acidified water (2.5-3.0 pH) in
pre-filled water bottles (300 ml, Aquavive®/Innovive) were provided ad
libitum. The bottle was equipped with a cap which had a hole with a
diameter of 1.2 mm (Figure 1B). The animal room was set on a 12:12 h
light:dark cycle (light on 7.00 to 19.00), room temperature was
maintained at 22 ± 2 °C with approximately 15 air changes per hour and
with relative humidity of 52-55 %. After one week of acclimation, mice
were handled daily for a week to minimize stress during blood
sampling. Mice were removed from the cage with cupped hands or the
cage ladder. The same techniques were used by the technical staff at
weekly cage changes.
Nine females and nine males of the same strain were paired at the age
of seven weeks. The hydration parameters were examined before mating
and at peak lactation after the females had their second litter, since
female mice usually have smaller first litters. When the first litter
was born all the pups except two were removed and these pups and the
males stayed with the females and were removed after the birth of the
second litter. The age of the females was 16 to 19 weeks when the
second litter was born.
Experimental design
Female mice were assigned to one of three groups according to a
completely randomized factorial design with two strains and three
watering alternatives. The watering alternatives were:
1. Water bottle (Standard, n=6)
2. Water bottle plus one water gel sachet (Gel, n=6)
3. Water bottle plus a water bowl (Bowl, n=6)
Six female mice were left unmated (three SWISS and three B6), serving as controls. They were housed in groups of three in an Innocage® with a water bottle and no water supplements.
The gel and water bowl (Figure 1A) were added after the birth of the
second litter. The water gel sachet size was 15 x 8 cm and was
attached to the feeder by tape. The gel (NECTA H2O gel, Labodia,
Switzerland) contained 98.5% pure water. The rest of the gel consisted
of gums, ascorbic acid and sodium benzoate. Before putting the gel
into the cage, one corner of the sachet was cut to make the gel
accessible to the mice. The metal water bowl was attached to the
feeder to avoid overturning. It was washed and filled with fresh tap
water every other day. As the pups got older they were able to jump up
on the bowl and drink from the water.
Blood sampling and analyses
For sampling, the vena saphena was punctured with a lancet and the
blood collected, maximum 8 ml/kg BW in total, in serum and plasma
vials (Microvette® CB 300 µl Serum, red US code and Microvette® CB 300
µl Lithium Heparin, green US code, Sarstedt, Nümbrecht Germany). The
serum samples were left for one hour and then centrifuged for 10
minutes at 3000 rpm. As indicators of hydration status, total plasma
protein (TP), hemoglobin (Hb), hematocrit (Htc), serum osmolality
(Osmol) and blood urea nitrogen (BUN) were measured before mating
(Pre) and at lactation day 14 (Peak). Magnesium (Mg) was also measured
because previous cases of lethal intestinal pseudo obstruction in peak
lactating female mice (Feinstein et al. 2008) had been diagnosed with
hypermagnesemia (Tjäder et al. 2013). Blood was collected from control
mice at the same time points.
Heparinized whole blood was analyzed with VetScan® VS2plus (Large
Animal Profile reagent rotor, SweVet ABAXIS, Triolab AB, Mölndal,
Sweden) and ABL90 FLEX (Radiometer, Bronshoj, Denmark). Serum
osmolality was analyzed with Fiske Micro-Osmometer Model 210 (Fiske
Associates, Norwood, Massachusetts, USA).
Other measurements
Water bottles were weighed on the first day after birth, before and
after the weekly change of water bottles, and at weaning. Litter size
and body weight of the pups and females were recorded at weaning.
Statistical analysis
InVivoStat was used for analyses (InVivoStat 1.3, 2020). Data was
checked for normally distributed residuals with normal probability
plots and for homogeneity of variance with predicted vs. residuals
plots. Baseline data (Pre) were compared between strains by Student’s
t-test or Mann-Whitney test. Hydration parameters at Pre and Peak were
analyzed using two-way repeated measures (RM) mixed model ANOVA, with
group as main factor, time point as repeated factor and strain as
blocking factor. Planned post hoc comparisons were performed without
adjustment for multiplicity (LSD test) between timepoints within
groups and between groups at Peak. Non-normally distributed data was
compared between strains and groups at Peak by Mann-Whitney or
Kruskal-Wallis test, and between time points within groups with
Wilcoxon signed rank test. Consumption of bottle water during
lactation was compared using a two-way ANOVA with strain and group as
factors. The comparison included both the total water volume consumed,
as well as the volume (ml) relative to body weight (g) of female and
litter. A p<0.05 was set as the level for significance.
Ethics approval
The study was approved by the regional animal ethics committee in
Lund, permit 5.8.18-17840/2017.
Results
Baseline
Table 1 shows baseline values before mating (Pre). SWISS mice had a
higher level of TP than B6 (p=0.01). No other parameter differed
between strains. TP levels were high in SWISS mice compared with
reference values. Osmol values were high in both strains compared with
reference values for mice. Some mice had higher levels of Mg than
reference values.
Table 1. Data for hydration parameters and magnesium in blood in females of two mouse strains before mating (Pre). Data presented as mean ± SD (min-max). Reference values are presented for comparison.
|
Parameter at baseline (Pre) |
RjOrl:SWISS (n=9) |
C57BL/6JRj (n=9) |
p-value (t-test) |
Reference values* |
Total protein (g/L) |
66 ± 5 (58-79) |
61 ± 3 (53-65) |
0.01 |
44-58 |
Hemoglobin (g/L) |
164 ± 9 (145-175) |
160 ± 11 (129-170) |
0.3 |
110-183 |
Hematocrit (%) |
50 ± 3 (45-54) |
49 ± 3 (39-52) |
0.4 |
32-52 |
|
Serum osmolality (mOsmol/kg) |
335 ± 9 (322-346) |
336 ± 13 (319-364) |
0.9 |
307-336 |
|
Blood urea nitrogen, BUN (mg/L) |
194 ± 39 (140-250) |
224 ± 49 (170-340) |
0.1 |
193-434 |
Magnesium (mmol/L) |
1.3 ± 0.3 (1.05-1.87) |
1.3 ± 0.1 (1.08-1.40) |
0.5 |
1-1.3 |
*Chollet et al. 2000; Otto et al. 2016; Santos et al. 2016; Serfilippi et al. 2003; Silverstein et al. 1961; Waymouth. 1970
Effect of lactation
Data at peak lactation are displayed in Table 2.
Strain (p=0.03) and time point (p<0.001) had a main effect on TP
whereas group did not (p=0.3, two-way RM ANOVA). There was an
interaction between group and time point (p=0.02). All lactating
groups had lower TP at Peak than Pre, whereas Control did not differ
(Standard: p=0.01, Gel: p=0.004, Bowl: p<0.001, Control: p=0.7). At
Peak, all groups had lower TP than Control (Standard: p=0.006, Gel:
p=0.01, Bowl: p=0.009).
Hb and Htc were not affected by strain (p=0.2), time point (p=0.5 and
p=0.6 respectively) or group (p=0.7, two-way RM ANOVA). Osmol was not
affected by strain (p=0.09) or group (p=0.2), but by time point
(p=0.03). Bowl had a lower Osmol at Peak than Pre (p=0.006), whereas
Standard (p=0.4), Gel (p=0.3) and Control Osmol did not change (p=0.8,
two-way RM ANOVA).
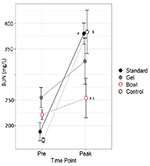
Strain (p=0.9) and group (p=0.2) had no effect on BUN, whereas time
point had an effect (p<0.001, two-way RM ANOVA). There was an
interaction between group and time point (p=0.01). Control and
Standard had higher levels of BUN at Peak than Pre (p<0.001)
whereas Gel and Bowl had not (p=0.1 and 0.5). BUN was lower in Bowl
than in Standard (p=0.005) and in Control (p=0.004) at Peak.
Strain and Group had no effect on Mg (p=1 and 0.4, respectively,
two-way RM ANOVA), whereas time point had an effect (p=0.002). Mg was
lower at Peak than Pre in Bowl and Control (p=0.01 and p=0.02,
respectively). Groups did not differ at Peak.
Table 2. Data for hydration parameters and magnesium in blood in females of two mouse strains [RjOrl:SWISS and C57BL/6JRj ] at peak lactation. Watering alternatives for lactating females: Standard = water bottle; Gel = water bottle + water gel sachet; Bowl = water bottle + water bowl. Control = non-lactating females, water bottle. Data presented as median (min-max), or mean ± SD (min-max).
|
Parameter at peak lactation |
Standard (n=6) |
Gel (n=6) |
Bowl (n=6) |
Control (n=6) |
Total protein (g/L) |
54 ± 4 (58-69) |
55 ± 4 (50-61) |
55 ± 6 (47-63) |
63 ± 8 (57-78) |
Hemoglobin (g/L) |
158 ± 11 (145-172) |
160 ± 10 (145-171) |
155 ± 14 (137-171) |
162 ± 10 (145-173) |
Hematocrit (%) |
48 ± 4 (44-53) |
49 ± 3 (44-52) |
47 ± 4 (42-52) |
59 ± 3 (44-53) |
|
Serum osmolality (mOsmol/kg) |
325 ± 14 (308-348) |
329 ± 13 (313-343) |
328 ± 8.7 (319-341) |
333 ± 8.0 (322-346) |
|
Blood urea nitrogen, BUN (mg/L) |
380 ± 51 (320-440) |
326 ±101 (170-410) |
254 ± 86 (190-400) |
383 ± 103 (320-440) |
Magnesium (mmol/L) |
1.1 ± 0.9 (1.1-1.2) |
1.1 ± 0.8 (1.0-1.2) |
1.1 ± 0.1 (0.9-1.3) |
1.1 ± 0.1 (1.0-1.2) |
Bottle water consumption and litter data
The absolute water consumption data was Log 10 transformed prior to
analysis (two-way ANOVA). Strain had an effect on bottle water
consumption; SWISS mice drank more than B6 (p<0.001). Group also
had an effect on consumption (p<0.001). There was no interaction
between strain and group (p=0.3). Mice in group Bowl drank less from
the bottle than Gel and Standard (p<0.001). Gel and Standard did
not differ (p=0.3).
When bottle water consumption was related to body weight of the female
and the litter, the consumption was instead higher in B6 than in SWISS
mice (p=0.002), and there was a main effect of group (p<0.001) as
well as an interaction between strain and group (p=0.02, two-way
ANOVA, non-transformed data). In both SWISS and B6 mice, water
consumption was lower in Bowl than Standard (p=0.004 and p< 0.001,
respectively) and was lower than Gel (p=0.01 and p<0.001,
respectively).
A higher number of pups per female were weaned from SWISS [13 (3-17),
n=9)] than B6 mice [5 (3-12), n=9, p< 0.001, Mann-Whitney test].
There was no difference in the number of pups weaned between groups
(Standard 10, Gel 7.5, Bowl 11.5, p=1.0, Kruskal Wallis test). SWISS
pups weighed more [21.5 (14.3-28.5g)] than B6 pups [12.4 (10.6-16.7g),
p<0.001, Mann-Whitney test]. Average pup weight, at four weeks of
age, was higher in Bowl (19 ± 5g) and Gel (18 ± 6g) compared with
Standard (14.5 ± 4g, p=0.008 and 0.02, respectively, two-way ANOVA).
|
Figure 2. Total protein levels before (Pre)
and at peak lactation in RjOrl:SWISS and C57BL/6JRj mice with
different types of watering: standard water bottle (Standard),
bottle plus water gel (Gel) or bottle plus water bowl (Bowl).
Non-lactating mice were used as control. N=6 per group. Data are
means ± SEM (§§ = p<0.05, @@, ## = p<0.01, two-way RM
ANOVA, post hoc test LSD). Click image to enlarge |
|
Figure 3. Osmolality before (Pre) and at peak
lactation in RjOrl:SWISS and C57BL/6JRj mice with different
types of watering: standard water bottle (Standard), bottle plus
water gel (Gel) or bottle plus water bowl (Bowl). Non-lactating
mice were used as control. N=6 per group. Data are means ± SEM
(§§ = p<0.01, two-way RM ANOVA, post hoc test LSD). Click image to enlarge |
|
Figure 4. Blood Urea Nitrogen (BUN) before
(Pre) and at peak lactation in RjOrl:SWISS and C57BL/6JRj mice
with different types of watering: standard water bottle
(Standard), bottle plus water gel (Gel) or bottle plus water
bowl (Bowl). Non-lactating mice were used as control. N=6 per
group. Data are means ± SEM (§§, ## = p<0.01, two-way RM
ANOVA, post hoc test LSD). Click image to enlarge |
|
Figure 5. Magnesium before (Pre) and at peak
lactation in RjOrl:SWISS and C57BL/6JRj mice with different
types of watering: standard water bottle (Standard), bottle plus
water gel (Gel) or bottle plus water bowl (Bowl). Non-lactating
mice were used as control. N=6 per group. Data are means ± SEM
(§§, ## = p<0.01, two-way RM ANOVA, post hoc test LSD). Click image to enlarge |
|
Figure 6. Bottle water consumption during lactation in absolute volume (A) and relative to body weight of female and litter at weaning (B) in two strains of mice: RjOrl:SWISS (n=9) and C57BL/6JRj (n=9). Standard= water bottle, Gel= water bottle plus a water gel sachet, Bowl= water bottle plus a water bowl. A: SWISS mice consumed more bottle water than B6 across groups (p<0.001) and mice in Bowl consumed less than Gel and Standard in both SWISS (§§, ## = p<0.05) and B6 mice (@@,$$= p < 0.01).
B: Water consumption/g was higher in B6 than
SWISS mice in Standard and Gel (p<0.05 and p<0.001,
respectively) and Bowl was lower than Standard and Gel in both
SWISS (@@, $$ = p<0.01) and B6 (§§, ## = p< 0.001, two-way
ANOVA; post hoc LSD). Individual data and means ± SEM. Click image to enlarge |
Discussion
The present study was undertaken to establish if female mice are dehydrated during peak lactation, when water is provided in bottles, and if additional provision of water in a bowl or as gel has any effect on hydration parameters. Two strains of female mice were examined during their second lactation, as female mice usually have smaller first litters (Brown et al. 1999).
According to the measured blood, plasma and serum parameters, neither
SWISS nor B6 mice were dehydrated before mating, or during peak
lactation. However, adding a water bowl to lactating mice led to a
marked reduction (54%) of bottle water consumption. The differences
were most pronounced if water consumption was related to body weight.
Mice showed no interest in ingesting the water gel, but rather
manipulated it and spread it around the cage, which prevented the
measurement of ingested amount. The bottle water consumption was very
similar in group Gel and Standard, and in both cases was higher than
in Bowl.
Lactation requires a very high energy intake for mice. It has been
estimated that in relation to body weight, a mouse produces about 16
times more milk per day than a cow (Hanwell 1977). At peak lactation,
a four to five-fold increase in feed intake is required, and mice need
to drink over 60 % of their body weight (Murai et al. 2013). Even if
drinking from a bottle may be a restriction, the data shows that
female mice keep a physiological water balance during peak lactation.
In a study where non-lactating mice were exposed to chronic water
restrictions of up to 50% of the daily ration for 8 days, none of the
hydration related variables (Osmol, Htc, TP) showed a significant
change from controls, even though mice were clearly thirsty and lost
10 % of their body weight (Bekkevold et al. 2013) . This indicates
that mice are equipped to deal with water restriction, which is likely
a physiological adaption for survival inherited from their wild
ancestors. Lactating mice that were fed a high potassium chloride
supplement during lactation increased their water intake by 100%,
which indicates a capacity to substantially increase water intake, but
with the cost of a 15 % reduction in body weight of the females and 15
% less body weight gain of the pups (Murai et al. 2013).
It was not possible to measure the water intake from the bowl, since
evaporation, splashing and contamination with bedding occurred.
Nevertheless, the reduced intake from the bottle indicates that mice
prefer to drink water from a bowl, which is in line with data from a
similar pilot study in fancy mice, in which the decreased bottle water
intake was even larger (67 %). Pet rabbits have been shown to prefer
drinking, and to drink faster and more, from open dishes compared with
nipple drinkers (Tschudin et al. 2011). This has led to the
recommendation to provide rabbits with water in open dishes, since
reduced water intake is linked to urinary tract disease. Horses show a
strong preference for drinking water from a bucket compared with an
automatic water bowl, even when the automatic flow rate approximates
to their normal drinking speed (Nyman and Dahlborn 2001).
There was further evidence from measured blood parameters that
lactating mice with access to a water bowl drank more; BUN was 34 %
lower in Bowl at peak lactation compared with both Standard and
Control, and only in Bowl was plasma osmolality lower (5 %) at peak
lactation compared with Pre.
In a study in Wistar rats, the changes in plasma osmotic pressure
during lactation were examined and it showed that total protein, Htc
and plasma Osmol were reduced during lactation, in comparison to dams
that had the pups removed at birth (Suzuki et al. 1993). In the
present study, no effect on Htc was seen and the Osmol did not differ
from controls, although in group Bowl, it was lower at Peak than Pre.
The decrease of plasma Osmol in the rats was explained by
hydro-dilution, which supports the suggestion that the mice in the
present study drank more with a bowl present and thereby diluted the
blood.
Total plasma protein was lower in all lactating mice compared with
control, on average the total plasma protein was 13 % lower during
lactation. This has been seen in several domestic species where plasma
proteins, and especially albumin, decrease during lactation (Eckersall
2008) and in one study with CD1 mice the albumin concentration in
serum was shown to be reduced by 50 % or more during peak lactation
(Monks and Neville 2004). Lactating female mice produce high protein
content milk as well as an increase in protein synthesis in the
mammary glands during lactation (Millican et al. 1987).
Magnesium levels did not differ between groups at peak lactation,
although mice in group Bowl had lower levels compared with Pre. We
included Mg in the analyses to investigate whether lactating mice had
higher levels of Mg during peak lactation, because a previous study
showed that mice which died from paralytic ileus during peak lactation
had increased Mg serum, with levels up to 6.5 mmol/L (Tjäder et al.
2013). Hypermagnesemia is an uncommon finding in mammals, since excess
magnesium is normally excreted by the kidneys, but has been shown to
cause paralytic ileus in humans (Golzarian et al. 1994; Izdes et al.
2008). Restricted water intake during lactation could possibly lead to
an accumulation of magnesium, which is absorbed by passive diffusion,
and cause a reduction of peristalsis of the gut (Glozarian et al.
1994). Mouse feed has been shown to contain up to 7 times the
recommended amount of magnesium for lactating females (Wise and
Gilburt 1981; National Research Council 1995).
Four weeks old pups weighed more in group Bowl (mean weight: Bowl 19
g; Gel 18 g; Standard 15 g) and pup weight was related to number of
pups in the litter. The median number of pups was however higher (non
significant) in group Bowl than Standard or Gel (11.5, 10 and 7.5,
respectively). Pups start eating solid food at day 16-17 postpartum
(Pritchett and Taft 2007) and since water and feed intake are closely
linked, easy access to water may have increased the solid feed intake
in both pups and females. It could also indicate that the females with
the water bowl produced more milk.
The present study was initially planned with twice the number of mice,
divided into two blocks. The second block was never performed because
the results showed that none of the 18 lactating mice in the first
block showed evidence of dehydration. It was therefore decided not to
carry out the second block, in accordance with the 3Rs. Since most
parameters were not affected by strain, the data from both strains
could be pooled, which made the groups large enough to establish clear
effects of water supplementation. There is a high number of
influencing factors for many clinical chemistry plasma analytes in
mice which makes it difficult to define universally valid normal
values (Otto et al. 2016). The presented data are however mostly in
the range of published levels for mice. Factors such as diet, housing,
blood sampling procedure, pre-analytical handling of samples,
equipment and methods used for analyses are known to affect the
results.
In summary, the study shows that mice do not suffer from dehydration
during peak lactation. Mice however prefer to drink, and seem to drink
more water, from a bowl than from a bottle.
Acknowledgement
Torvald och Britta Gahlins Foundation
Conflict of interest
The authors declared no potential conflict of interest.
References
- Bekkevold, C.M., Robertson, K.L., Reinhard, M.K., Battles, A.H., Rowland, N.E., (2013). Dehydration parameters and standards for laboratory mice. Journal of the American Association for Laboratory Animal Science. 52(3), 233-239.
- Brown, R.E., Mathieson, W.B., Stapleton, J., Neumann, P.E., (1999). Maternal Behavior in Female C57BL/6J and DBA/2J Inbred Mice. Physiology & Behavior. 67(4), 599-605. doi: 10.1016/s0031-9384(99)00109-2.
- Chollet, D., Franken, P., Raffin, Y., Malafosse, A., Widmer, J., Tafti, M., (2000). Blood and brain magnesium in inbred mice and their correlation with sleep quality. American Journal of Physiology. Regulatory, Integrative and Comparative Physioliology. 279(6), R2173-2178.
- Eckersall, D.P., (2008). Chapter 5 - Proteins, Proteomics, and the Dysproteinemias. In: Kaneko JJ, Harvey JW, Bruss ML, ed. Clinical Biochemistry of Domestic Animals. 6th ed. San Diego: Academic Press.
- Feinstein, R.E., Morris, W.E., Waldemarson, A.H., Hedenqvist, P., Lindberg, R., (2008). Fatal acute intestinal pseudoobstruction in mice. Journal of the American Association for Laboratory Animal Science. 47(3), 58-63.
- Hedenqvist,P., Holmberg, Å., Jensen-Waern, M., (2015). Are lactating mice deprived of water? A pilot study of water intake. Joint HSBLAS-ESLAV-ECLAM Meeting; Athens, Greece: HBLAS, p. 32.
- Golzarian, J., Scott, H.W. Jr, Richards, W.O., (1994). Hypermagnesemia-induced paralytic ileus. Digestive diseases and sciences. 39(5), 1138-1142. doi: 10.1007/BF02087570.
- Hanwell, A., (1977). Physiological effects of lactation on the mother. In: Peaker, M., (ed.). Comparative aspects of lactation, Symposia of the zoological society of London 41. London: Academic Press.
- Izdes, S., Kesimci, E., Kanbak, O., (2008). Paralytic ileus as a complication of iatrogenic hypermagnesaemia without renal dysfunction. Anaesthesia and Intensive Care. 36(1), 124.
- Mähler, M., Berard, M., Feinstein, R., Gallagher, A., Illgen-Wilcke, B., Pritchett-Corning, K., Raspa, M., (2014). FELASA recommendations for the health monitoring of mouse, rat, hamster, guinea pig and rabbit colonies in breeding and experimental units. Laboratory Animals. 48, 178-192. doi: 10.1177/0023677213516312.
- Millican, P.E., Vernon, R.G., Pain V.M., (1987). Protein metabolism in the mouse during pregnancy and lactation. Biochemical Journal. 248, 251-257. doi: 10.1042/bj2480251.
- Monks, J., Neville, M.C., (2004). Albumin transcytosis across the epithelium of the lactating mouse mammary gland. The Journal of Physiology. 560(1), 267-280. doi: 10.1113/jphysiol.2004.068403.
- Murai, I., Shukuin, S., Sugimoto, M., Ikeda, S., Kume, S., (2013). Effects of high potassium chloride supplementation on water intake and bodyweight gains in pregnant and lactating mice. Animal Science Journal. 84(6), 502-507. doi: 10.1111/asj.12025.
- National Research Council US, (1995). Nutrient Requirements of Laboratory Animals, 4th ed. Washington, DC: The National Academies Press. P. 192.
- Nyman, S., Dahlborn, K., (2001). Effect of water supply method and flow rate on drinking behavior and fluid balance in horses. Physiology & Behavior. 73(1-2), 1-8. doi: 10.1016/s0031-9384(00)00432-7.
- Otto, G.P., Rathkolb, B., Oestereicher, M.A., Lengger, C.J., Moerth, C., Micklich, K., Fuchs, H., Gailus-Durner, V., Wolf, E., Hrabĕ de Angelis, M., (2016). Clinical Chemistry Reference Intervals for C57BL/6J, C57BL/6N, and C3HeB/FeJ Mice (Mus musculus). Journal of the American Association for Laboratory Animal Science. 55(4), 375-386.
- Pritchett, K.R, Taft, R., (2007). Chapter 3 - Reproductive Biology of the Laboratory Mouse. In: Fox JG, Davisson MT, Quimby FW, Barthold SW, Newcomer CE, Smith AL, ed. The Mouse in Biomedical Research. 2nd ed. SBurlington: Academic Press. pp. 91-121.
- Santos, E.W., Oliveira, D., Hastreiter, A., Silva, G., Beltran, J., Tsujita, M., Crisma, A., Neves, S., Fock, R., Borelli, P., (2016). Hematological and biochemical reference values for C57BL/6, Swiss Webster and BALB/c mice. Brazilian Journal of Veterinary Research and Animal Science. 53(2), 138-145.
- Serfilippi, L.M., Pallman, D.R., Russell, B., (2003). Serum clinical chemistry and hematology reference values in outbred stocks of albino mice from three commonly used vendors and two inbred strains of albino mice. Contemporary Topics in Laboratory Animal Science. 42(3), 46-52.
- Silverstein, E., Sokoloff, L., Mickelsen, O., Jay, G.E., (1961). Primary Polydipsia and Hydronephrosis in an Inbred Strain of Mice. The American Journal of Pathology. 38(2), 143-159.
- Suzuki, K., Hirose, H., Hokao, R., Takemura, N., Motoyoshi, S., (1993). Changes of plasma osmotic pressure during lactation in rats. The Journal of Veterinary Medical Science. 55(4), 561-564. doi: 10.1292/jvms.55.561.
-
Tjäder, S., Waldemarsson, A., Feinstein, R., Hedenqvist, P., (2013).
Acute Fatal Insterstinal Pseudo-Obstruction (IPO) in Lactating
Female Mice. ESLAV-ECLAM conference, Cambridge2013, UK.
Tschudin, A., Clauss, M., Codron, D., Hatt, J.M., (2011). Preference of rabbits for drinking from open dishes versus nipple drinkers. Veterinary Record. 168(7), 190. doi: 10.1136/vr.c6150. - Waymouth, C., (1970). Osmolality of mammalian blood and of media for culture of mammalian cells. In Vitro. 6(2), 109-127.
- Weijnen, J.A., (1998). Licking behavior in the rat: measurement and situational control of licking frequency. Neuroscience & Biobehavioral Reviews. 22(6), 751-760. doi: 10.1016/s0149-7634(98)00003-7.
-
Wise, A., Gilburt, D.J., (1981). Variation of minerals and trace
elements in laboratory animal diets. Laboratory Animals. 15(4),
299-303. doi: 10.1258/002367781780952834.