Original scientific article
Using cage ladders as a handling device reduces aversion and anxiety in laboratory mice, similar to tunnel handling
by Rebecca Sandgren1*, Charlotta Grims1, John Waters2, Jane L Hurst2*
1Biomedical Service division, Medical Faculty, Lund
University, Sweden
2Mammalian Behaviour & Evolution Group, Institute of Infection, Veterinary and Ecological Sciences, University of Liverpool, UK
Correspondence: Rebecca Sandgren, Biomedical Service division, Medical Faculty,
Lund University, Sölvegatan 19, 221 84 Lund, Sweden, E-mail:
rebecca.sandgren@med.lu.se
Jane Hurst, Mammalian Behaviour & Evolution Group, Institute of
Infection, Veterinary and Ecological Sciences, University of
Liverpool, Leahurst Campus, Neston CH64 7TE, UK E-mail:
jane.hurst@liverpool.ac.uk
Summary
Handling laboratory animals for husbandry and other procedures can be
an important source of anxiety and stress, compromising animal welfare
as well as the reliability of research that is sensitive to background
stressors. Studies have revealed that picking up laboratory mice by
the tail induces aversion, anxiety, physiological stress and
depression-like behaviour, but such negative responses can be reduced
substantially by using a handling tunnel that mice enter readily with
minimal familiarisation. It has not been tested whether anxiety and
aversion can be reduced similarly by using other objects to lift up
mice from their home cage. Here we compared the willingness of
C57BL/6NRj mice to interact voluntarily with their handler after being
picked up either on a plastic ladder present in the home cage, or
inside a familiar tunnel, or lifted by the base of the tail and then
returned to the home cage. We also tested anxiety in open field and
elevated plus maze tests once animals were familiarised with their
assigned handling method. While mice picked up briefly by the tail
were unwilling to interact with the hand that picked them up, mice
picked up by ladder or tunnel readily approached, climbed on or
entered these devices, with no significant difference in time spent
with ladder or tunnel. Anxiety in an unfamiliar open field was reduced
to a similar extent in ladder and tunnel handled mice compared with
those picked up by the tail. Mice handled by tunnel also showed
reduced anxiety in an elevated plus maze compared to those handled by
tail, while ladder handling resulted in an intermediate response. Our
study shows that, like tunnels, using home cage ladders to pick up
mice reduces anxiety and avoids the aversion that is induced by
picking up mice by their tails. We discuss the potential practicality
of using ladders and tunnels to handle mice in different contexts.
Introduction
Mice are the most common vertebrate species used in research and are subjected to frequent manipulations and handling in captivity. It is well recognized that routine handling can cause aversion, anxiety and stress, especially when laboratory mice are picked up by the tail (Balcombe et al. 2004; Hurst and West 2010; Gouveia and Hurst 2013, 2017; Ghosal et al. 2015). The aversion and anxiety induced can influence both the behaviour and physiology of mice as well as compromising animal welfare (Brown and Winnicker 2015; Bailey 2017). Recently, two alternative methods have been developed that can be used to pick up mice instead of tail handling: the mouse can be guided into a handling tunnel and then lifted up for delivery to the hand, transferred between cages or to test apparatus; or mice can be scooped up on the open hand without restraint, in a method known as cupping, once they are familiar with being lifted (Hurst and West 2010; Gouveia and Hurst 2019). Mice picked up in a tunnel or on the open hand will interact voluntarily with their handler while those picked up by tail avoid contact, reflecting a major difference in the aversiveness of these handling methods as reported for different laboratories, handlers and mouse strains (Hurst and West, 2010; Gouveia and Hurst 2013, 2017, 2019; Clarkson et al. 2018, 2020; Nakamura and Suzuki 2018; Henderson et al. 2020a; Sensini et al. 2020). Mice picked up by the tail also show greater anxiety in standardised tests compared to those picked up by one of these non-aversive methods (Hurst and West 2010; Gouveia and Hurst 2013, 2019; Clarkson et al. 2018, 2020; Nakamura and Suzuki 2018; Henderson et al. 2020a; Sensini et al. 2020), and have elevated measures of physiological stress such as increased urination and defecation during handling (Hurst and West 2010; Nakamura and Suzuki 2018; Henderson et al. 2020a), higher plasma corticosterone in response to a stressful situation (Ghosal et al. 2015) and enlarged adrenal glands indicative of chronic stress (Clarkson et al. 2020). Furthermore, mice picked up by the tail show behaviour indicative of a depressive-like state, with reduced sucrose consumption anhedonia (Clarkson et al. 2018), greater immobility in a forced swim test and reduced burrowing behaviour (Sensini et al. 2020) compared to those picked up in a handling tunnel.
Minimising handling anxiety in laboratory animals is essential as this
both negatively impacts animal welfare and can be an important source
of unwanted variation and poor reliability and replicability in many
areas of animal research (Brown and Winnicker 2015; Bailey 2017). For
example, picking up mice by the tail can increase blood glucose stress
responses and reduce glucose tolerance in diabetes research (Ghosal et
al. 2015), eliminate reliable exploration of test stimuli in cognitive
behavioural tests (Gouveia and Hurst 2017), increase variability in
pharmacological testing (Nakamura and Suzuki 2018), and reduce
responsiveness to the rewards that are used to train animals in a
range of behavioural and cognitive tasks (Clarkson et al. 2018)
compared to the use of non-aversive handling methods. Importantly, the
positive effects of using non-aversive methods (rather than capturing
and lifting mice by the tail) persist even when mice experience
restraint by the tail or scruff (Hurst and West 2010; Gouveia and
Hurst 2019), subcutaneous (Gouveia and Hurst 2019) or intraperitoneal
(Henderson et al. 2020a) injections, oral gavage (Nakamura and Suzuki
2018), anaesthesia (Henderson et al. 2020a), or marking by tattoo or
ear-tagging (Roughan and Sevenoaks 2018) once picked up. Thus,
although mice are not physically restrained when picked up by tunnel
or cupping, they are tolerant of restraint when this is required for
health inspections or other procedures.
When working with animals, the handling method not only needs to
minimise any aversion, anxiety and stress in animals but also needs to
be efficient and secure from a practical perspective so that it is
workable in daily routines. While both cupping and handling tunnels
are non-aversive to mice once they are familiar with these methods,
mice require more time to become familiarised with cupping (Hurst and
West 2010; Gouveia and Hurst 2019). Further, cupping without physical
restraint is not suitable for very young mice or strains that are very
jumpy, or for inexperienced handlers who are less able to judge when
an unrestrained mouse might jump off the open hand unless the mice
concerned are very placid (Gouveia and Hurst 2019). However, mice are
easily and securely picked up inside a handling tunnel (tube) once
handlers are trained in this technique, while the very brief handling
necessary to transfer mice between cages at cage cleaning
(approximately two seconds) is sufficient to familiarise mice with
this method and achieve positive effects (Gouveia and Hurst 2017,
2019). By contrast, while picking up mice by tail is secure, even
brief and infrequent tail handling during cage cleaning induces strong
aversion (Gouveia and Hurst 2019), tail handled mice are more
difficult to handle and restrain, and handlers are at an increased
risk of being bitten (Nakamura and Suzuki 2018; Gouveia and Hurst
2019). Handling tunnels overcome these issues but, nonetheless,
perceived incompatibility of tunnels with a caging system,
experimental apparatus, or implants carried by animals might be
deterrents for using handling tunnels routinely in some facilities
(Henderson et al. 2020b). This led us to explore other handling
devices that might be as effective as tunnels for minimising aversion,
anxiety and stress in laboratory mice while also being efficient and
secure.
A major difference between picking up mice by tunnel or cupping versus
the tail, besides direct restraint, is that animals face the handling
device head-on and step into the tunnel or onto the hand themselves
(even though guided to do so by the handler) rather than being grasped
from behind and pulled up backwards. This may lead to a perception of
greater choice and cooperation that might also be achieved by using
other objects that animals walk on or into. Gouveia and Hurst (2013)
recommended using home cage tunnels where possible, allowing mice to
become highly familiar with tunnels that also provide enrichment in
the home cage. However, to our knowledge, no studies have looked at
whether other enrichment objects could also be used for non-aversive
handling, such as different types of shelter or items added to
encourage activity and increase the use of space in a cage that would
otherwise be unavailable. In this study, we compare the responses of
mice picked up with a home cage ladder (Figure 1A) with those of mice
picked up in a familiar tunnel or by the base of the tail, to
establish whether handling using an enrichment device such as a ladder
is also non-aversive and as effective as tunnel handling for reducing
anxiety in laboratory mice. Our animal facility at Lund University
designed and developed the ladder used in this study to provide mice
with the opportunity for climbing (Roemers et al. 2019) in cages that
do not have bars. The ladders provide a familiar grid that mice
readily climb on and grip, potentially providing a suitable device to
pick up mice from the cage. The ladder is angled upwards at one end
(Figure 1A,C) which makes it easier to scoop up a mouse. We predicted
that mice picked up on their home cage ladder would be less anxious
than those picked up by tail and readily interact with the ladder,
with a positive response equal to that of mice picked up in a home
cage tunnel.
|
Figure 1. Standard enrichment in mouse cages
at Lund University (A). Placement of handling device during the
test of voluntary interaction with the handler for tunnel (B),
ladder (C) and tail (D) methods. Click image to enlarge |
Materials & Methods
Animal use and care was in accordance with EU directive 2010/63/EU. All procedures were approved by Malmö-Lund regional ethics committee, ethical permit number: 5.8.18-02982/2020.
Animals and housing conditions
48 C57BL/6NRj mice (24 females, 24 males) were obtained from Janvier
Labs (Le Genest-Saint-Isle, France) at four weeks of age. On arrival,
they were individually identified by ear punch and divided into 24
cages with two same-sex mice per cage. Microbiological health
monitoring was carried out according to FELASA guidelines (Mähler et
al. 2014) with negative results.
All mice where housed in disposable IVC cages (Innocage®outside
dimensions 37.3 x 23.4 x 14.0 cm; Innovive, San Diego, USA) at Lund
University, Sweden. Each cage contained corncob bedding, nesting
material (InnorichmentTM, Innovive, San Diego, USA) and had been
irradiated. Irradiated pelleted food (A40-SP25, SAFE, France) and
acidified water (2.5-3.0 pH) in pre-filled water bottles (300 ml,
Aquavive®/Innovive, SanDiego, USA) were provided ad libitum.
Animals were maintained on a 12:12 h light:dark cycle (lights on at
07:00), at 22 ± 2 °C with approximately 15 air changes per hour and
52-55 % relative humidity. An aspen gnaw stick (Tapvei Estonia OÜ,
Harjumaa, Estonia), a plastic ladder (Mouse climber, 200 x 60 mm
polyethylene terephthalate, Innovive, San Diego, USA) and an opaque
plastic tunnel (100 mm x 50 mm diameter polypropylene, local retail,
Sweden) were provided as additional cage enrichment (Figure 1).
Cages were assigned randomly to one of three handling methods (tail,
tunnel or ladder) using random numbers, with four female and four male
cages per method (16 mice per handling group). Mice were handled only
by their assigned method from arrival. Cages were arranged in the cage
rack such that handling method and sex were distributed in a balanced
design. For the first three weeks, mice were acclimatized to the
laboratory and cages were opened only if food required replenishment.
Cages were changed once during the study, with mice placed into a
clean cage after the first voluntary interaction test. All handling
and testing were carried out during the light phase of the circadian
cycle.
Handling methods
To simulate routine handling of mice at cage change, all lifts were
for approximately two seconds then mice were lowered back into their
cage. Mice assigned to tail handling were grasped at the base of the
tail between thumb and forefinger by a gloved hand and gently lifted
into the air. Mice handled by tunnel were gently guided by a gloved
hand into their home cage tunnel and then lifted into the air without
covering the ends of the tunnel. For ladder handling, mice were guided
gently onto the ladder by a gloved hand and lifted into the air. The
mice were handled by two female handlers alternating between days. For
anxiety tests, all mice were handled by one of the two handlers.
Gloves (Powder-free orange nitrile glovesTM, SHIELDskinTM, SHIELD
Scientific, ND Bennekom, Netherlands) were disinfected (Contec®
ProChlor, Vita Verita AB, Kungsängen, Sweden) between cages and
allowed to air dry, and all enrichments were removed from the cage
prior to handling. In each handling session, the order of cages was
randomised but the males were always handled first as female scents
can promote increased androgen levels and competitive aggression
within male groups (Koyama 2004) and then gloves changed before
handling females to avoid any impact of male scents on females. The
first mouse picked up in each cage was alternated between daily
handling sessions. When mice were transported to test arenas by tail,
their body weight was supported, and then lifted by the tail
unsupported for delivery to the arena.
Handling and test schedule
The study started when mice were seven weeks old. Mice were briefly
handled once per day on week days (Monday-Friday) for nine handling
sessions. Voluntary interaction with the handler was tested
immediately after handling sessions 1, 5 and 9 (days 1, 5 and 11). An
open field test was conducted on day 15 or 16 from the start of the
study, and an elevated plus maze on day 17 or 18. Gloves were worn
during all tests and disinfected between mice.
Voluntary interaction with handler
We carried out tests of voluntary interaction with the handler
immediately after both mice in a cage had been handled in sessions 1,
5 and 9. The device used to handle the animals (gloved hand holding a
home cage tunnel, gloved hand holding a home cage ladder, or gloved
hand only for tail handled animals) was introduced into the home cage
and held on the substrate without movement for 60s (Figure 1B-D). The
session was video recorded and transcribed using BORIS event logging
software (Friard and Gamba 2016). The total time when none, one or
both mice in the cage interacted with the hand, tunnel or ladder
(close sniffing or physical contact, including the gloved hand holding
a tunnel or ladder) was recorded. These data were then used to
calculate the proportion of test time spent in voluntary interaction
per mouse averaged over both mice in the same cage.
Open field test
To assess anxiety in an open field test, mice were picked up by their
assigned method and delivered to an unfamiliar open field arena (40 x
40 x 40 cm opaque black walls, Stoelting Co., Illinois, USA) for a 5
min test. As anxious animals are reluctant to spend time in open areas
(Gould et al. 2009), we measured time spent in the central 7.5 x 7.5
cm zone of the arena using a video tracking system (ANY-maze,
Stoelting Co., Illinois, USA). Cages, and mice within cages, were
tested in random order, with no difference in test order between
treatments (Kruskal-Wallis H2 = 1.14, p = 0.57). The
test arena was disinfected using ethanol and wiped off with water
between mice.
Elevated plus maze test
Anxiety was also assessed in an elevated plus maze consisting of two
closed arms (35 x 5 cm) with 15 cm high side walls and two open arms
(35 x 5 cm) with no side walls, connected by a central hub (5 x 5 cm),
elevated 60 cm above ground and constructed of grey painted steel.
Mice were picked up and delivered to the maze by their assigned method
for a 5 min test. Cages, and mice within cages, were tested in random
order, with no difference in test order between treatments
(Kruskal-Wallis H2 = 0.37, p = 0.83). A video
tracking system (ANY-maze, Stoelting Co., Illinois, USA) recorded the
number of entries and total time spent on the open arms of the maze as
measures of anxiety (Pellow et al. 1985; Walf and Frye 2007), and the
number of entries to the closed arms and total distance moved on the
maze as measures of general activity.
Data analysis
Statistical analyses were carried out using SPSS version 25 (IBM
software). Shapiro-Wilks tests and qqnorm plots checked for any
deviation of residuals from normality for each parametric model, using
data transformation where appropriate. The raw data analysed in this
study are provided in Supplementary Table 1. One male mouse from the
tunnel group died before the Open Field and Elevated Plus Maze tests
were performed. Also, one male mouse from the tail group was not
recorded in the Open field test due to technical problems.
The total time spent in voluntary interaction with the handler at each
time point was calculated as a proportion of the 60s test and averaged
across the two mice in the same cage to reflect the lack of
independence of animals tested together. A repeated measures ANOVA
analysed the effects of handling method and sex on time spent
interacting with the handling device in tests repeated after handling
sessions 1, 5 and 9. As the duration of voluntary interaction across
sessions lacked sphericity, a Greenhouse-Geisser correction was
applied when assessing the effect of session.
To assess anxiety in the open field test, a repeated measures ANOVA
assessed the effects of handling method and sex on the proportion of
test time spent in the inner zone of the open arena, using cage as a
within-subjects factor to account for lack of independence between
mice in the same cage. Data were log transformed to meet assumptions
of parametric analysis. To assess anxiety in the elevated plus maze,
repeated measures ANOVAs assessed the effects of handling method and
sex on the number of entries and total time on the open arms of the
maze, using cage as a within-subjects factor. A similar analysis
assessed the number of entries to closed arms as a measure of general
activity, while non-parametric Kruskal-Wallis and Mann-Whitney tests
assessed the effects of handling method and sex respectively on the
total distance moved during the test. As problems with recording meant
that some tests were slightly shorter than 5 min (elevated plus maze
test duration 289 ± 3s), all data were adjusted to an equivalent rate
for a 5 min test.
Where a significant effect of handling method was found, Bonferroni
post hoc comparisons checked which methods differed significantly from
each other. Throughout, p < 0.05 was regarded as statistically
significant.
Results
Voluntary interaction with handler
We compared the willingness of mice to interact voluntarily with their
assigned handling device immediately after they had been picked up
briefly by the device (a gloved hand holding a tunnel or ladder, or a
gloved hand only for mice picked up by the tail). This was assessed as
the amount of time spent in close interaction (close sniffing or
physical contact) during a 60 s test, averaged over both mice in a
cage. Voluntary interaction differed substantially according to the
handling device in both sexes (F2,18 = 129.6, p < 0.0001; Figure
2). This difference was evident from the first handling session and
did not change significantly over handling sessions 1, 5 and 9 (effect
of session: F1.4, 36 = 1.56, p = 0.23; interaction between method and
session: F2.7, 36 = 0.38, p = 0.75; Figure 2). While mice spent very
little time interacting with the hand after being picked up by the
tail (2.9 ± 0.3 s per 60 s test), those picked up by tunnel or ladder
spent much longer interacting with their handling device (20.3 ± 0.7 s
per test). There was no significant difference in the duration of
interaction between ladder and tunnel (Bonferroni post hoc comparison,
p = 1.0; Figure 2). There was also a qualitative difference in the
type of interaction shown: while tail handled mice only sniffed the
hand and rarely made paw contact, those handled by ladder or tunnel
readily climbed on the handling devices, went inside the tunnel and
made paw contact with the gloved hand that held the ladder or tunnel.
|
Figure 2. The effect of brief handling by
different methods on voluntary interaction with the handling
device (mean ± sem per cage). Mice were picked up for
approximately 2 s (similar to a cage transfer) by the tail
(pink), home cage tunnel (blue) or ladder (green) in nine daily
handling sessions. Voluntary interaction was assessed after
handling on first, fifth and ninth session (% of 60 s test
sniffing or contacting the device, averaged over both mice in
the same cage). N = 8 cages per method (four male, four female).
P values from a repeated measures ANOVA across the three
repeated tests. Different letters (a,b) indicate significant
differences between methods from Bonferroni post hoc comparisons
(p < 0.0001). Click image to enlarge |
Anxiety
Handling method influenced anxiety in an open field test, assessed as
the amount of time spent in the inner zone of the unfamiliar arena
(F2,16 = 25.4, p < 0.0001). While males spent more time in the
inner zone overall compared to females (F1,16 = 6.7, p = 0.02),
handling method had a similar effect on both sexes (interaction
between sex and method: F2,16 = 1.20, p = 0.33). Mice handled by
either tunnel or ladder spent much more time in the inner zone than
those handled by tail, with no significant difference between tunnel
or ladder (Bonferroni post hoc comparison, p = 0.69; Figure 3A).
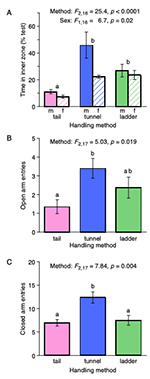
Handling method also influenced the number of entries to the open arms of an elevated plus maze (F2,17 = 5.03, p = 0.019), with a similar response in both sexes (F1,17 = 0.76, p = 0.40; interaction between sex and method: F2,17 = 4.35, p = 0.24). Post hoc comparisons indicated that mice picked up by the tail made significantly fewer entries to the open arms than those picked up in a tunnel (Bonferroni test, p = 0.011, Figure 3B), consistent with greater anxiety among tail handled mice. Mice picked up on a ladder showed an intermediate response that did not differ significantly from either tunnel or tail handled mice (Figure 3B). The mean time that mice spent on the open arms followed a similar pattern (tail: 10.4 ± 2.8 s; tunnel: 19.6 ± 5.8 s; ladder: 15.3 ± 2.7s per cage) but this did not differ significantly between handling methods (F2,17 = 1.90, p = 0. 18) as only female mice picked up in a tunnel tended to spend more time on the open arms than those handled by other methods (interaction between method and sex, F2,17 = 2.90, p = 0. 08).
As measures of general activity in the elevated plus maze test, we
also examined the number of entries into closed arms and the total
distance moved on the maze during the test. Handling method
significantly influenced the number of closed arm entries (F2,17 =
7.84, p = 0. 004; Fig. 3C) but not the total distance moved during the
test (Kruskal-Wallis H2 = 3.30, p = 0.19). Mice handled by tunnel made
more entries to the closed arms of the maze than those handled by tail
(Bonferroni p = 0.004) or ladder (Bonferroni p = 0.009), although they
did not travel a greater total distance during the test. This suggests
that tunnel handled mice were more active in exploring both the open
and closed arms of the maze but were not generally more active
overall.
|
Figure 3. Responses of mice handled by different methods in open field and elevated plus maze tests of anxiety (mean ± sem). After being picked up for approximately 2 s over nine daily sessions to familiarise mice with their designated handling method (pink: tail, blue: tunnel, green: ladder), mice were tested in an open field test (A) and an elevated plus maze test (B,C). Mice picked up using a tunnel or ladder spent more time in the inner zone of an open field, indicating reduced anxiety compared to those picked up by tail (A). Entries to the open arms of an elevated plus maze were more frequent in tunnel compared to tail handled mice but intermediate in ladder handled mice (B). Tunnel handled mice also entered closed arms more frequently than tail or ladder handled mice (C). P values from repeated measures ANOVAs with mice from the same cage as a within-subjects factor (time in inner zone of an open field log transformed to meet assumptions of parametric analysis). Different letters (a,b) indicate significant differences between methods from Bonferroni post hoc comparisons.
Click image to enlarge |
Discussion
In agreement with our predictions, mice picked up on a home cage
ladder voluntarily approached and climbed on the ladder immediately
after handling, showing the same lack of aversion towards the ladder
and hand holding the ladder that mice show towards the tunnel. By
contrast, those picked up by tail avoided contact with the
handler’s gloved hand. Mice picked up on a ladder also showed
reduced anxiety in an unfamiliar open field compared to tail handled
mice, spending a very similar length of time in the open inner zone as
those handled by tunnel. However, measures of anxiety in ladder
handled mice in an elevated plus maze test were intermediate between
tunnel and tail handled animals.
When testing voluntary interaction immediately after handling, the
level of aversion shown by tail handled mice was very similar to that
shown in previous studies (Hurst and West 2010; Gouveia and Hurst
2013, 2017, 2019; Clarkson et al. 2018, 2020; Nakamura and Suzuki
2018; Henderson et al. 2020a; Sensini et al. 2020). When tail handled
mice did approach, they only sniffed the hand and rarely made paw
contact, as described previously (Hurst and West 2010; Sensini et al.
2020), with an observed tendency to also stay close to the cage walls.
By contrast, both tunnel and ladder handled mice moved around freely
and were more explorative in the cage, climbing on or in these devices
and making contact with the gloved hand. These findings are in
accordance with the behaviour patterns that differentiate anxious from
non-anxious behaviour in mice (Simon et al.1994; Prut and Belzung
2003). In our study, the voluntary interaction test was designed to
assess whether mice found the handling device itself aversive after
handling, so we did not test how mice picked up by ladder responded
when only the handler’s gloved hand was presented as for tail
handling. However, other studies have shown that mice picked up by
tunnel interact readily with just a gloved hand as well as with the
tunnel (Hurst and West 2010; Roughan and Sevenoaks 2018; Henderson et
al. 2020a). Given the similarity of response between tunnel and ladder
handled mice, and their willingness to make paw contact with the hand
holding the ladder, it is likely that mice picked up with a ladder
will respond in the same positive way that tunnel handled mice do when
they need to be manipulated and restrained in the hand (Hurst and West
2010; Gouveia and Hurst 2019).
Mice interacted freely with the tunnel or ladder right from their
first brief handling session. By contrast, mice scooped up by cupping
on the open hand require familiarisation through multiple handling
sessions before they interact freely with the gloved hand that has
just picked them up (Hurst and West 2010; Gouveia and Hurst 2019).
Several factors may influence this immediate positive response to
ladder handling. First, mice were highly familiar with climbing on the
ladder in their home cage. However, prior familiarity may only play a
minor role given that familiarity with handling tunnels in the home
cage slightly improves interaction in a first handling session, but
mice also respond positively to handling tunnels even when these are
completely unfamiliar (Gouveia and Hurst 2013). Secondly, the rigid
surface of ladders and tunnels may be preferred by animals as these
will provide much less variable surface movement when mice are lifted
up compared to a hand. Thirdly, mice will be more familiar with rigid
plastic surfaces compared to the material, warmth and odour of gloves
(López-Salesansky et al. 2015). Lastly, the ladder grid is designed
for easy gripping, which may provide a greater perception of safety
when mice balance on a moving ladder compared to the smooth moving
surface of a gloved hand, while mice in tunnels are protected by the
encompassing tunnel walls. All of these factors combined may help to
attract mice more readily to tunnels and ladders than to the hand, and
to reduce anxiety as mice are lifted such that initial familiarisation
is less necessary than for cupping on the open hand.
Time spent in the inner zone of an open field suggests that mice
handled by ladder experienced similar low levels of anxiety as tunnel
handled animals, with both moving freely around the arena, while mice
picked up by tail showed high anxiety and were reluctant to enter the
inner zone as reported previously (Gouveia and Hurst 2017, 2019;
Clarkson et al. 2018, 2020; Nakamura and Suzuki 2018; Henderson et al.
2020a). In our tests, males generally spent more time in the inner
zone of the open field compared to females, suggesting that males were
less anxious or bolder during exploration. However, both sexes showed
a similar reduction in time spent in the inner zone after tail
handling compared to those handled by either ladder or tunnel. Sex
differences have been reported previously in some measures of anxiety,
although whether males or females show greater anxiety varies between
strains and between studies (Augustsson et al. 2005; An et al. 2011).
In C57BL/6J mice, An et al. (2011) found no sex difference in time
spent in the inner zone of an open field but did not report the
handling method used. By contrast, when examining the effects of
handling by tail or tunnel on anxiety in an open field among C57BL/6J
mice, Gouveia and Hurst (2019) report very similar results to our
study, with males spending more time overall in the central zone while
both sexes spent less time in the inner zone when picked up by tail
compared to those picked up in a tunnel. In the elevated plus maze
test, mice of both sexes handled by tail also showed significantly
increased anxiety as evidenced by fewer entries onto open arms
compared to tunnel handled mice, which was consistent with previous
reports (Hurst and West 2010; Gouveia and Hurst 2013, 2019; Ghosal et
al. 2015; Clarkson et al. 2018, 2020; Nakamura and Suzuki 2018;
Henderson et al. 2020a). However, those handled by ladder showed an
intermediate response and did not move as freely around the maze as
tunnel handled mice. In this test, males and females did not differ
overall in the number of entries to the open arms, in agreement with
Gouveia and Hurst (2013), where tail handled mice of both sexes made
fewer entries onto open arms compared to tunnel handled mice. Other
studies have found more entries to open arms among C57BL/6J females
compared to males overall (Hurst and West 2010; Gouveia and Hurst
2019), suggesting that males of this strain may sometimes demonstrate
greater anxiety in this test than females, which is opposite to the
sex difference found in open field tests discussed above. This may
explain why only females handled by tunnel spent more total time on
the open arms as well as entering these arms more frequently, as these
were the least anxious animals in this test. However, regardless of
the specific test and measures used, all of these studies (including
the study reported here) have consistently found greater anxiety among
mice of both sexes picked up by the tail compared to those picked up
in a tunnel. Mice of both sexes picked up by a ladder were clearly
less anxious in the open field test than those picked up by the tail
and not significantly more anxious than tunnel handled mice in this
test. They also readily approached and interacted with the ladder,
which was similar to their response to handling tunnels and without
the aversion shown by tail handled mice, but effects on anxiety in the
elevated plus maze were less clear cut.
While conducting these tests, we noted a difference in the ease of
placing mice onto the elevated plus maze between the handling methods.
Use of a tunnel provided the smoothest delivery to the maze as mice
easily could be tipped out backwards onto the central hub. By
contrast, mice delivered to the maze on a ladder clung to the handling
device and sometimes had to be pushed off firmly, resulting in less
voluntary cooperation and a less precise placement. This problem did
not occur when handling animals in cages, where animals are very
familiar with the environment and readily leave the ladder. Nor did it
occur when delivering animals by ladder to the open field test,
suggesting that mice found the elevated plus maze a more unfamiliar
and potentially threatening environment to enter. We also found that
delivering mice to the elevated plus maze by tail was sometimes
difficult, given the very small central hub and the tendency of mice
held by the tail to twist. Some tail handled mice ran straight out
onto one of the open arms of the maze when released. This did not
appear to be a voluntary action to enter the open arms, but instead an
attempt to run away when the tail grip was released. Thus, delivery of
mice to this particular test by either tail or ladder may result in
potential confounding effects which can be avoided by using a handling
tunnel.
As the ladder is provided as standard enrichment in cages at our Lund
facility and frequently is used for handling, we have seen that it has
many practical advantages. As it is already in each cage, it is easily
accessible and provides a biosecure way to transfer mice into new
cages as shown for other handling devices (Doerning et al. 2018). We
have observed that mice are easy to inspect when on the ladder, making
it possible to see the mouse from all angles including the ventral
view without restraining the animal as it grips onto the ladder. When
opaque handling tunnels are used, such inspection is not possible
without tipping the animal out onto the hand, although animals can
usually be observed well through clear handling tunnels. Having the
mice already gripping on to the ladder also allows for scruffing
directly from the ladder. The construction of the ladder, as an
“open” device, may also make it particularly suitable to
pick up animals that have implants, surgical staples or similar
devices on their body. However, as the ladder provides an open
surface, there is a potential risk that jumpy young animals or strains
might jump off the ladder, which we have not investigated in this
study. However, we did not find that mice attempted to jump from the
ladder during our handling and tests and this has not been reported as
an issue in our animal facility where ladders are used routinely to
pick up mice from the cage, and it is possible to cover the mice with
a hand where this might be a risk. Although we have not collected data
systematically on the time it takes to transfer mice between cages
using ladders and other enrichment objects in the cage, we have
observed that using these non-aversive handling methods to transfer
mice instead of picking them up by the tail does not prolong the time
spent for cage change.
In this study we have shown that using a home cage ladder to handle
mice is non-aversive, similar to using a tunnel. Like tunnel handling,
this can also reduce anxiety and provides a refinement compared to
tail handling. However, tunnels might be better for transferring mice
in some contexts. Our findings suggest that mice can be picked up on
or in a variety of objects present in the cage, making handling easier
and avoiding aversion and high anxiety in the animals. This will
benefit animal welfare and the practicality of animal management, and
may also improve scientific reliability in animal models that are
susceptible to background stress and anxiety.
Acknowledgements
We are grateful to the animal caretakers, and R.S. and C.G. also thank their managers at Lund University for their assistance and support.
Conflict of interest
The authors declared no potential conflict of interest.
References
- An, X.-L., Zou, J.-X., Wu, R.-Y., Yang, Y., Tai, F.-D., Zeng, S.-Y., Jia, R., Zhang, X., Liu, E.-Q., Broders, H., (2011). Strain and sex differences in anxiety-like and social behaviors in C57BL/6J and BALB/cJ mice. Experimental Animals. 60, 111-123. doi: 10.1538/expanim.60.111
- Augustsson, H., Dahlborn, K., Meyerson, B.J., (2005). Exploration and risk assessment in female wild house mice (Mus musculus) and two laboratory strains. Physiology & Behavior. 84, 265-277. doi: 10.1016/j.physbeh.2004.12.002
- Bailey, D.J., (2017). Does the stress inherent to laboratory life and experimentation on animals adversely affect research data? ATLA Alternatives to Laboratory Animals. 45, 299-301. doi: 10.1177/026119291704500605
- Balcombe, J.P., Barnard, N.D., Sandusky, C., (2004). Laboratory routines cause animal stress. Contemporary Topics in Laboratory Animal Science. 43, 42-51.
- Brown, M.J., Winnicker, C., (2015). Chapter 39 - Animal welfare. in Laboratory animal medicine: Third edition. pp 1653-1672. doi: 10.1016/B978-0-12-409527-4.00039-0
- Clarkson, J.M., Dwyer, D.M., Flecknell, P.A., Leach, M.C., Rowe, C., (2018). Handling method alters the hedonic value of reward in laboratory mice. Scientific Reports. 8, 2448. doi: 10.1038/s41598-018-20716-3
- Clarkson, J.M., Leach, M.C., Flecknell, P.A., Rowe, C., (2020). Negative mood affects the expression of negative but not positive emotions in mice. Proceedings of the Royal Society B: Biological Sciences. 287, 20201636. doi: http://dx.doi.org/10.1098/rspb.2020.1636
- Doerning, C.M., Thurston, S.E., Villano, J.S., Kaska, C.L., Vozheiko, T.D., Soleimanpour, S.A., Lofgren, J.L., (2018). Assessment of mouse handling techniques during cage changing. Journal of the American Association for Laboratory Animal Science. 58, 767-773. doi: 10.30802/AALAS-JAALAS-19-000015
- Friard, O., Gamba, M., (2016). BORIS: a free, versatile open-source event-logging software for video/audio coding and live observations. Methods in Ecology and Evolution.7, 1325-1330. doi: 10.1111/2041-210X.12584
- Ghosal, S., Nunley, A., Mahbod, P., Lewis, A.G ., Smith, E.P., Tong, J., D’Alessio, D.A., Herman, J.P., (2015). Mouse handling limits the impact of stress on metabolic endpoints. Physiology and Behavior. 150, 31-37. doi: 10.1016/j.physbeh.2015.06.021
- Gould, T.D., Dao, D.T., Kovacsics, C.E., (2009). The open field test. Neuromethods. 42, 1-20. doi: 10.1007/978-1-60761-303-9-1
- Gouveia, K., Hurst, J.L., (2013). Reducing mouse anxiety during Handling: Effect of experience with handling tunnels. PLoS ONE. 8, e66401. doi: 10.1371/journal.pone.0066401
- Gouveia, K., Hurst, J.L., (2017). Optimising reliability of mouse performance in behavioural testing: The major role of non-aversive handling. Scientific Reports. 7, 44999. doi: 10.1038/srep44999
- Gouveia, K., Hurst, J.L., (2019). Improving the practicality of using non-aversive handling methods to reduce background stress and anxiety in laboratory mice. Scientific Reports. 9, 20305. doi: 10.1038/s41598-019-56860-7
- Henderson, L.J., Dani, B., Serrano, E.M.N., Smulders, T.V., Roughan, J.V., (2020a). Benefits of tunnel handling persist after repeated restraint, injection and anaesthesia. Scientific Reports. 10, 14562. doi: 10.1038/s41598-020-71476-y
- Henderson, L.J., Smulders, T.V., Roughan, J.V., (2020b). Identifying obstacles preventing the uptake of tunnel handling methods for laboratory mice: An international thematic survey. PLoS ONE. 15, e0231454. doi: 10.1371/journal.pone.0231454
- Hurst, J.L., West, R.S., (2010). Taming anxiety in laboratory mice. Nature Methods. 7, 825-826. doi: 10.1038/nmeth.1500
- Koyama, S., (2004). Primer effects by conspecific odors in house mice: a new perspective in the study pf primer effects on reproductive activities. Hormones and Behavior. 46, 303-310. doi: 10.1016/j.yhbeh.2004.03002
- López-Salesansky, N., Mazlan, N.H., Whitfield, L.E., Wells, D.J., Burn, C.C., (2016). Olfactory variation in mouse husbandry and its implications for refinement and standardisation: UK survey of non-animal scents. Laboratory Animals. 50, 286-295. doi: 10.1177/0023677215614296
- Mähler, M., Berard, M., Feinstein, R., Gallagher, A., Illgen-Wilcke, B., Pritchett-Corning, K., Raspa, M., (2014). FELASA recommendations for the health monitoring of mouse, rat, hamster, guinea pig and rabbit colonies in breeding and experimental units. Laboratory Animals. 48, 178-192. doi: 10.1177/0023677213516312
- Nakamura, Y., Suzuki, K., (2018). Tunnel use facilitates handling of ICR mice and decreases experimental variation. Journal of Veterinary Medical Science. 80, 886-892. doi: 10.1292/jvms.18-0044
- Ono, M., Sasaki, H., Nagasaki, K., Torigoe, D., Ichii, O., Sasaki, N., Agui, T., (2016). Does the routine handling affect the phenotype of disease model mice? Japanese Journal of Veterinary Research. 64, 265-271. doi: 10.14943/jjvr.64.4.265
- Pellow, S., Chopin, P., File, S.E., Briley, M., (1985). Validation of open: closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. Journal of Neuroscience Methods. 14, 149-167. doi: 10.1016/0165-0270(85)90031-7
- Prut, L., Belzung, C., (2003). The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: A review. European Journal of Pharmacology. 463, 3-33. doi: 10.1016/S0014-2999(03)01272-X
- Roemers, P., Hulst, Y., van Heijningen, S., van Dijk, G., van Heuvelen, M.J.G., De Deyn, P.P., van der Zee, E.A., (2019). Inducing physical inactivity in mice: Preventing climbing and reducing cage size negatively affect physical fitness and body composition. Frontiers in Behavioral Neuroscience. 13, 221. doi: 10.3389/fnbeh.2019.00221
- Roughan, J.V., Sevenoaks, T., (2019). Welfare and scientific considerations of tattooing and ear tagging for mouse identification. Journal of the American Association for Laboratory Animal Science. 58, 142-153. doi: 10.30802/AALAS-JAALAS-18-000057
- Sensini, F., Inta, D., Palme, R., Brandwein, C., Pfeiffer, N., Riva, M.A., Gass, P., Mallien, A.S., (2020). The impact of handling technique and handling frequency on laboratory mouse welfare is sex-specific. Scientific Reports. 10, 17281. doi: 10.1038/s41598-020-74279-3
- Simon, P., Dupuis, R., Costentin, J., (1994). Thigmotaxis as an index of anxiety in mice. Influence of dopaminergic transmissions. Behavioural Brain Research. 61, 59-64. doi: 10.1016/0166-4328(94)90008-6
-
Walf, A.A., Frye, C.A., (2007). The use of the elevated plus maze as
an assay of anxiety-related behavior in rodents.
Nature Protocols. 2, 322-328. doi:
10.1038/nprot.2007.44