Technical report
A detachable tourniquet to aid rodent tail-vasculature catheterisation; especially useful for preclinical imaging
by Kasper Hansen1-4,*
1Department of Forensic Medicine, Aarhus University, 8200 Aarhus N,
Denmark.
2Department of Clinical Medicine (Comparative Medicine Lab), Aarhus
University, 8200 Aarhus N, Denmark.
3Section for Zoophysiology, Department of Biology, Aarhus
University, 8000 Aarhus C, Denmark.
4University of Leicester, East Midlands Forensic Pathology Unit,
Leicester Royal Infirmary, Leicester LE2 7LX, UK
Correspondence: Kasper Hansen
email: kaha@forens.au.dk
Summary
Rodents are frequently used animal models in biomedical research. Catheterisation of a blood vessel in the tail is often performed to enable intravascular access for administration of drugs, fluid or blood sampling. Many experimenters employ a “Minasian tourniquet” to dilate the vasculature in the tail in order to ease catheterisation. Certain experiments, e.g. those that employ preclinical imaging techniques, require relatively long catheter-tubing (~0.5 to 2 meters) to reach the rodent inside the scanner. Following catheter insertion, great care must be taken to carefully guide the loop of the tourniquet back along the entire length of the catheter. To minimise the risk of withdrawing the catheter accidentally during this step, a simple detachable tourniquet was created. This tourniquet can be removed completely and does not require careful guiding of the tourniquet-loop back along the catheter.
Introduction
Rodent species are widespread animal models in various fields of basic and translational biomedical research. Intravascular access for sampling blood, administration of anaesthetics or pharmaceuticals, and for minimal invasive preclinical imaging studies with contrast agents and/or tracers (Cunha et al. 2014), is a relatively common prerequisite for many rodent studies. In rodents, blood vessels in the tail are particularly useful sites for vascular access. This is because the tail is relatively easy to access and it is spaced apart from the body’s internal organs, which is especially convenient and relevant for isotope imaging to minimise potential spill-over effects of tracer from the injection site onto the imaging-signal from the organ of interest (Alstrup et al. 2018).
It is vital during the catheterisation process that the experimenter precisely localises the major veins (lateral or dorsal) before insertion of the needle/catheter, a procedure that requires both skill and experience (Groman and Reinhardt 2004). To make this easier experimenters often use different techniques to enhance the tail vessels. These include warming the tail under a heating lamp or immersing the tail in lukewarm water. However, a rubber band, piece of string or a simple tourniquet placed around the tail-base, which will raise the blood pressure and therefore distend the veins (Weiss et al. 2000), is probably one of the most effective and therefore widespread methods to aid rodent tail catheterisation.
A simple tourniquet, devised for venepuncture by Harvey Minasian (1980), is a very helpful and widely used tool for tail vein catheterisation in rodents. The Minasian tourniquet (MT) is designed from ubiquitously available and cheap standard laboratory components: a syringe and a piece of suture/thread. The MT is very easy to use but for studies utilising longer catheter tubing, e.g. preclinical imaging studies where relatively long catheter tubing (> 0.5 to several meters) is typically needed to reach the animal inside the scanner, the MT has one critical disadvantage: once the catheter is fitted it is obligatory to guide the loop of the MT along the entire length of the catheter tube. This increases the risk of accidentally pulling out the catheter.
Here I describe a simple modification to the MT, allowing safe, fast
and full detachment/removal of the “detachable tourniquet”
(DT) which eliminates the need to guide the tourniquet-loop along the
catheter tube.
Materials & Methods
A DT can be constructed solely from cheap standard laboratory materials (Figure 1A). The plunger of a standard 1.0 mL plastic syringe was completely retracted from the syringe barrel. A thread (e.g. a piece of thick suture) was cut at a length approximately equal to the length of the plunger (Figure 1A). A hole was pierced in the plunger’s rubber seal with an injection needle and one end of the thread was pulled through the hole and tied to the tip of the plunger; ideally using a knot that tightens under load (e.g. a slipknot as displayed in Figure 1a). The loose end of the suture was then guided through the syringe’s opening/tip as the plunger was reinserted into the syringe barrel. A fastener-ring, which is used to fasten the thread during DT employment, can be easily constructed from various standard laboratory materials (see details in the legend of Figure 1B-F).
|
Figure 1. The DT and some useful standard
laboratory materials for its construction. A) The thread is
tied to the plunger using a slipknot that tightens under load
(a) and threaded through the pierced rubber seal of the plunger.
B-F) A fastener-ring (FR) can be constructed from e.g. B) the
tip of an injection needle, C) a piece of a pipette-tip cut to
appropriate dimensions, D) the opening of a syringe-tip
compatible container, E) a piece of tubing with an inner
diameter of ~4 mm, and/or F) the cap of a three-way stopcock
after using a sharp 5.5 mm drill to create a hole. G) The
fastener-ring is used to squeeze the thread against the
syringe-tip, providing enough friction to withstand the pull of
the carefully retracted plunger, which tightens the thread
around the rodent tail (a 5 mm diameter silicone tube was used
to simulate the tail of a rodent). Click image to enlarge |
The DT is employed in the following order:
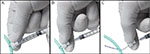
- The loose end of the thread is threaded through the fastener-ring, around the tail and back around the fastener-ring, which is then used to squeeze the thread tightly against the tip of the syringe (Figure 1G; Figure 2A)
- The plunger is gently retracted to carefully raise the tension of the thread around the tail before inserting the intravascular catheter according to procedures described in detail elsewhere (Weiss et al. 2000). The friction of the rubber seal against the syringe barrel will maintain thread tension during the procedure.
- Complete detachment of the tourniquet can be achieved by pressing the plunger back into the syringe barrel and then removing the fastener-ring from the syringe-tip (Figure 2B-C)
- The thread can be safely diverted away from inserted catheter and tail, which allows safe removal of the DT (Figure 2C).
|
Figure 2. The DT and some useful standard
laboratory materials for its construction. A) The thread is
tied to the plunger using a slipknot that tightens under load
(a) and threaded through the pierced rubber seal of the plunger.
B-F) A fastener-ring (FR) can be constructed from e.g. B) the
tip of an injection needle, C) a piece of a pipette-tip cut to
appropriate dimensions, D) the opening of a syringe-tip
compatible container, E) a piece of tubing with an inner
diameter of ~4 mm, and/or F) the cap of a three-way stopcock
after using a sharp 5.5 mm drill to create a hole. G) The
fastener-ring is used to squeeze the thread against the
syringe-tip, providing enough friction to withstand the pull of
the carefully retracted plunger, which tightens the thread
around the rodent tail (a 5 mm diameter silicone tube was used
to simulate the tail of a rodent). Click image to enlarge |
Results and Discussion
The detachable tourniquet was constructed using ubiquitously available standard laboratory materials and simple tools providing no additional production costs. The standard laboratory components used in this description need not be strictly followed but may serve as inspiration.
The MT is supposedly used successfully in laboratories worldwide (interference from the author's personal communication with preclinical imaging-researchers of various nationalities). No formal data on the DT’s performance and safety were systematically collected because the DT is produced by a simple modification to the MT, which does not affect its way of providing tension to the tail. There have been no reported accidents or otherwise unwanted effects during or following the use of the DT in approximately 50 cases known to the author. Removing the tourniquet after catheterisation is more complicated with the MT compared to the DT. Therefore, the described DT may, compared to the MT, lower the risk of accidental withdrawal of an inserted catheter and potentially ultimately reduce the number of experimental rodents needed.
Conclusion
Studies with model animals should follow the 3R’s: replacement, reduction and refinement (Russell and Burch 1959, Baumans 2004). The proposed DT-design fulfils the two later R’s of this ethical principle.
Acknowledgements
Kristian Bang provided all the high-quality photographs used in the manuscript. I also want to sincerely thank Aage Kristian Olsen Alstrup (Department of Nuclear Medicine & PET Centre, Department of Clinical Medicine, Aarhus University) for his constructive critique of the manuscript.
Conflict of interest
The author declared no potential conflict of interest.
References
- An, X.-L., Zou, J.-X., Wu, R.-Y., Yang, Y., Tai, F.-D., Zeng, S.-Y., Jia, R., Zhang, X., Liu, E.-Q., Broders, H., (2011). Strain and sex differences in anxiety-like and social behaviors in C57BL/6J and BALB/cJ mice. Experimental Animals. 60, 111-123. doi: 10.1538/expanim.60.111
- Alstrup, A.K., Munk, O.L, Landau, A.M., Lillethorup, T.P., (2018). PET radioligand injection for pig neuroimaging. Scandinavian Journal of Laboratory Animal Science. 44(2), 1-5. doi: https://doi.org/10.23675/sjlas.v44i0.509.
-
Baumans, V., (2004). Use of animals in experimental research: an
ethical dilemma? Gene Therapy. 11(S1),
S64-S66. doi: 10.1038/sj.gt.3302371.
-
Cunha, L., Horvath, I., Ferreira, S., Lemos, J.,
Costa, P., Vieira, D., Veres, D.S., Szigeti, K.,
Summavielle, T., Máthé, D., Metello, L.F., (2014).
Preclinical imaging: an essential ally in modern biosciences.
Molecular Diagnosis & Therapy. 18(2),
153–173. doi: 10.1007/s40291-013-0062-3.
-
Groman, E.V., Reinhardt, C.P., (2004). Method to quantify tail vein
injection technique in small animals.
Journal of the American Association for Laboratory Animal
Science. 43(1), 35–38.
-
Minasian, H., (1980). A simple tourniquet to aid mouse tail
venipuncture. Laboratory Animals. 14(3),
205–205. doi: https://doi.org/10.1258/002367780780937571.
-
Russell, W.M.S., Burch, R.L., (1959).
The Principles of Humane Experimental Technique. London,
UK: Methuen.
Weiss, J., Taylor, G.R., Zimmermann, F., Nebendahl, K., (2000). Chapter 25 - Collection of body fluids, in Krinke, G.J., (ed.) The Laboratory Rat. London: Academic Press (Handbook of Experimental Animals), pp. 485–510.