Original scientific article
The adjuvant-induced rat model of monoarthritis: welfare implications
and possible refinement strategiess
by *Mie S. Berke and Klas S.P. Abelson
Department of Experimental Medicine, Faculty of Health and Medical
Sciences, University of Copenhagen
Correspondence: Mie Schou Berke
Department of Experimental Medicine
Blegdamsvej 3B, DK-2200 Copenhagen N, Denmark
E-mail: mibe@sund.ku.dk
Tel. +45 93 56 55 66
Summary
This study investigated welfare, mechanical hyperalgesia and model specific parameters (mobility, stance, joint stiffness and ambulation impairment) during a three-week period in a rat model of monoarthritis. The objective was to identify possible targets for refinement of the model and consequently improve animal welfare. Monoarthritis was induced in eight male Sprague-Dawley rats by an intra-articular injection of 20 µL Complete Freund’s Adjuvant (CFA containing heat-killed Mycobacterium tuberculosis) in the left tibio-tarsal joint. The effects on subjects were monitored daily and compared to a negative control group. The induced monoarthritic response consisted of pronounced acute inflammation observed as joint swelling, redness and temperature rise in the injected hind leg. Several parameters such as bodyweight, mobility, stance, stiffness and ambulation were also affected. All CFA-injected rats demonstrated a significantly higher nociceptive response in an electronic von Frey test throughout the study, with a markedly lower mechanical threshold in the first three days after injection. Complete Freund’s Adjuvant was sufficient to induce monoarthritis in all CFA-injected rats. It is concluded that more attention should be paid to alleviating pain in the acute phase where the animal’s well-being appears to be most compromised, and that an injection volume of 20 µL CFA is sufficient to induce monoarthritis in the tibio-tarsal joint.
Introduction
Animal models investigating inflammatory joint disease mechanisms and anti-arthritic agents have been used extensively for decades. Adjuvant-induced arthritis (AIA) models are among models used frequently, particularly in identification and validation of new therapeutic agents (Choudharyet al. 2018). The CFA AIA model is readily inducible with only a single injection of heat-killed Mycobacterium tuberculosis in oil, has great reproducibility and shares common symptoms with the human disease Rheumatoid Arthritis (RA) (Kim and Moudgil 2009; Choudharyet al. 2018). There are several other models of RA, both genetic -and induced models, but none mimic human RA completely (Choudhary et al. 2018). CFA has mainly been injected intradermally or subcutaneously at the base of the tail or in the footpad, which has caused widespread and multifactorial systemic disease (Pearson and Wood 1959; Waksman et al. 1960; Pearson 1963). CFA-induced polyarthritis induces organism-wide abnormal changes inflicting severe distress and pain in the animals. Acute inflammation is seen at the site of injection within hours whereas the chronic reaction, with inflammation and hyperalgesia of multiple joints (ankle, wrist, tarsal, carpal, interphalangeal and spinal joints), occurs from 10-14 days after CFA injection (Waksman 2002). The primary lesion is synovitis, followed by periarthritis, peritendinitis and periostitis, pannus formation with destruction of cartilage and bone, and lastly ankylosis. Secondary changes involve oedema, fibrin deposition and necrosis with granulocytes, followed by proliferation of synoviocytes and fibroblasts, stimulation of osteoblasts and osteoclasts, bone destruction and remodelling (Waksman 2002).
A less severe model of chronic joint inflammation, the CFA
monoarthritis model, was introduced by Butler etal. (1992) in an
attempt to refine the polyarthritis model. This modified model was
originally induced by an intra-articular injection of CFA into the
tibio-tarsal joint (Butler et al. 1992) but several joints have since
been investigated to model monoarthritis (Wuet al. 1998; Imbe et al.
2001). The CFA-induced tibio-tarsal method models arthritis in one
joint, characterized by joint inflammation, cartilage destruction and
bone erosion, which persist for several weeks (up to 6 weeks), and
where the affected limb shows a marked increase in sensitivity to
pressure. Compared to the polyarthritis model, animals gain weight,
remain active, and display little systemic disturbance (Butleret al.
1992; Donaldsonet al. 1993; Hashmiet al. 2010), which indicates that
animal welfare is less compromised in the monoarthritic model.
Despite the reduction of negative welfare effects compared to the
polyarthritis model, relatively little attention has been paid to
further refinement of the monoarthritic rat model. It is evident that
the induction of monoarthritis is painful to the animal. Even though a
certain degree of pain and inflammation may be necessary for studying
the pathogenesis and progression of the disease, all unnecessary pain
and distress should be avoided. Hence, there is a need to refine the
monoarthritis model in order to minimize any potential suffering.
The purpose of this study was to identify potential refinement
strategies during a three-week period in the monoarthritic rat model
induced with CFA intra-articularly in the tibio-tarsal joint. The
impact of 20 µL CFA injected in the tibio-tarsal joint on animal
welfare, mechanical hyperalgesia and model specific parameters was
investigated and compared to untreated control animals.
It was hypothesized that a 20-µL CFA injection into the tibio-tarsal joint would produce a localized and valid inflammatory joint disease of mild to moderate severity during the three weeks of the study. The study aimed to identify the parameters most affected at certain time points, in order to determine the welfare implications of the model, and thus provide a basis for future refinement.
Materials and Methods
Ethics statement. The experiments were carried out at the Department of Experimental Medicine, University of Copenhagen, in an AAALAC accredited animal facility in accordance with the Guide for Care and Use of Laboratory Animals (Institute of Laboratory Animal Research 2011) and with Directive 2010/63/EU (2010). All animal experimentation was approved by the Danish competent authority, the Animal Experiment Inspectorate under the Danish Ministry of Environment and Food (license number 2014-15-0201-00257).
Animals and housing. Sixteen male NTac:SD rats, obtained from Taconic (Ry, Denmark) weighing approximately 200 g on arrival, were used in the study. After one week of acclimatization, the rats were approximately eight weeks of age when testing commenced. The age was determined according to literature on the AIA model (Bolonet al. 2011). Only male rats were used, since male rats manifest more severe arthritic characteristics providing useful measureable pain-evoked behaviours (Cai et al. 2006). AIA susceptibility differs among various outbred and inbred rat strains. The Sprague-Dawley (SD) strain was chosen for the study because it is a commonly used outbred AIA-susceptible rat strain (Kim and Moudgil 2009) which has a high incidence, with low variability, of clinical signs and has similar arthritis features to the commonly used inbred Lewis rat AIA model as well as human RA (Cai et al. 2006).
Animals were housed in pairs in type IV S individually ventilated
cages (size 480 x 375 x 210 mm) (Tecniplast, Varese, Italy). Cages
were equipped with aspen chip bedding (Tapvei, Kortteinen, Finland),
paper nesting material (Lillico, Horley, United Kingdom), wooden
sticks (Tapvei), and polycarbonate rat retreats (Molytex, Glostrup,
Denmark) and cardboard tunnels (Lillico) as environmental enrichment.
The room temperature was kept at 22 °C, with a relative humidity of
55% and a 12 h light/dark cycle, with lights on between 6:00 and
18:00, with 15-30 min of dimmed light at transitions. The rats were
given pelleted food (Altromin 1314; Altromin GmbH & Co., Germany)
and tap water ad libitum.
Study design. Fifteen rats were randomly allocated in pairs of two (cage level) to two groups. The two groups consisted of seven rats as a non-CFA-injected control group and eight rats injected with CFA. The control group were injected with saline in the tibio-tarsal joint. One rat from the control group was excluded from the study, because of a wound acquired before initiation of the study. Group sizes were determined using the resource equation method (Mead 1988; Festing and Altman 2002; Festing and Weigler 2003), which indicated six to eight experimental units per group. The experimenter was blinded to the group allocation throughout the study.
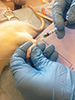
Induction of monoarthritis. Complete Freund’s adjuvant (CFA) containing heat-killed and dried Mycobacterium tuberculosis (strain H37Ra, ATCC 25177) was purchased from Sigma-Aldrich (St. Louis, MO, USA). The procedure was performed under brief isoflurane anesthesia (Attane Vet, Isoflurane 1000 mg/g, ScanVet) with 3% isoflurane delivered in pure oxygen at a flow rate of 1.0 L/min in an induction chamber until loss of righting occurred. The anaesthetized rat was then moved to a polystyrene tray, where a nose cone was applied. The rat was placed in right lateral recumbency and the pedal withdrawal reflex was tested before injection. When the site of injection was found, an insulin syringe (29 G) was inserted intra-articularly into the tibio-tarsal joint and 20 µL of CFA (or saline for the control animals) was injected (Figure 1). The experimenter was blinded and the injection procedures were done by the same experimenter throughout the study in order to avoid inter-experimenter deviations.
|
Figure 1. Intra-articular injection technique.
The figure shows a syringe containing green food colour and CFA,
used in training of the technique. The injection point was
located by palpating the fossa of the lateral malleolus of the
fibulae on a slightly flexed and pronated left ankle. An insulin
syringe (29 G) was inserted intra-articularly into the
tibio-tarsal joint and 20 µL of CFA (or saline for the control
animals) was injected when a distinct loss of resistance was
felt (approximately 2 mm). Click image to enlarge |
Animal welfare assessment (WA) and humane endpoints. In order to measure pain- related behavior and general wellbeing, animals were individually monitored daily for 15 min in their home cages, using a welfare score sheet adapted from Hampshire et al. (2001), which is shown in Table 1. This particular score sheet was selected since it involves welfare indicators (general appearance, porphyrin staining, gait and posture, wounds and body weight changes) that are readily and reliably recognizable, can be scored for severity and are relevant to this species and model. All observations were done blinded and by the same experimenter. The overall WA score was also used in determining the humane endpoint. If the score exceeded 0.4, the animal would be euthanized.
Table 1: Welfare assessment score sheet adapted from Hampshire et al., 2001. Each animal was assigned a score in each category. The individual scores were summed for an overall welfare assessment score for each individual.
|
General appearance |
Bright and alert |
|
Burrowing or hiding, quiet but rouses when touched |
|
Burrowing or hiding, quiet but rouses when touched. No exploration when lid off, burrows, hides, head presses. Might be aggressive when touched |
|
Porphyrin staining |
None |
Mild |
Obvious on face or paws |
|
Gait and posture |
Normal |
|
Mild incoordination when stimulated, hunched posture, mild piloerection |
|
Obvious ataxia or head tilt, hunching, severe piloerection |
|
Body weight |
Up to 5% weight loss |
5-10% weight loss |
10-20% weight loss |
|
Wounds |
|
Bites or scratches itself, leading to wounds |
Electronic von Frey test. Mechanical hypersensitivity was assessed using electronic von Frey (EVF) testing (model EVF3 with a hard tip from Bioseb, Vitrolles, France) and was measured before CFA-injection (pre-induction), as well as on days 3, 7, 10, 14, 17 and 21. The rats were placed on an elevated metal grid platform in clear acrylic chambers (size 16.5 x 24.2 x 14.6 cm) with dark partition sidewalls to prevent visual contact between rats. Four rats were tested at a time, with an individual habituation time of 30 min before testing. The tip was applied from underneath through openings in the metal grid floor, with increasing force, perpendicular to the plantar surface, nearest the tibio-tarsal joint, of each hind paw. The mechanical threshold corresponds to the applied weight in grams required to elicit paw withdrawal. The test was repeated three times for each hind paw at an interval of two seconds, starting with the non-injured right paw and then the injured left paw. The EVF tip was only applied when the rat stood still in a natural position with all four paws placed on the surface and a paw withdrawal response was only recorded where there was a complete lifting of the stimulated hind paw.
Monoarthritic specific parameters. The pathological development of arthritis was evaluated twice weekly by measuring the joint circumference and using a score sheet, modified from Butler et al. (1992), for mobility, stance, impairment of ambulation and stiffness of the joint (Table 2). The animals were observed in their home cages between 9:00 and 12:00. The same person made all observations to avoid inter-observer variation. The joint circumference was measured with a digital calliper. The circumference (C) was calculated using the following formula:, where ‘a’ is the radius of the dorso-plantar axis and b is the radius of the medio-lateral axis (Taget al. 2016).
Table 2: Model specific parameters (modified from Butler et al., 1992). Each animal was assigned a score in each category.
|
Mobility |
Reference score |
The rat lies down only |
0 |
The rat crawls only |
1 |
The rat walks with difficulty |
2 |
The rat walks and runs with difficulty |
3 |
The rat walks and runs normally |
4 |
|
Stance |
|
The rat stands on three paws only |
0 |
|
The rat stands with the arthritic paw touching floor, toes curled under |
1 |
|
The rat stands bearing some weight on the arthritic limb |
2 |
|
The rat stands bearing weight equally on all four limbs |
3 |
|
Ambulation impairment |
|
Normal ambulation |
0 |
Mild, slight lame |
1 |
Moderate, toe touching ground |
2 |
Severe, limb carried |
3 |
|
Joint stiffness |
|
Normal |
0 |
|
Restriction of full range of flexion or extension |
1 |
|
Restriction of full range of flexion and extension |
2 |
Histopathology. The rats were euthanized on day 23. The legs
were removed by bilateral dissection proximal to the knee joint,
immediately skinned and fixed in formalin (10%) for seven days.
Subsequently, bones were decalcified in
ethylene-diamine-tetraacetic-acid (EDTA) solution for 14 days. The
decalcification solution was prepared by dissolving 100 g EDTA
disodium salt dehydrate (Merck Millipore, Burlington, USA) in 1 L of
0.1 M Tris buffer, and the pH was set to seven (titration with NaOH).
To speed up the decalcification process, the tissue samples were
subsequently washed in Tris Buffered Saline (TBS) for four days and
then further decalcified in formic acid citrate (formic acid 98%,
tri-sodium citrate, dihydrate) for another seven days. The
tibio-tarsal joints were trimmed before decalcification using a method
similar to Bolon et al. (2011). The decalcified legs were dehydrated
in ethanol (70%), cut longitudinally and mounted in cassettes. The
samples were then embedded in paraffin wax, cut in longitudinal
sections and stained with hematoxylin-eosin (HE).
The histopathological assessment was compromised by technical issues
involving incomplete cuts in the sample sections. This resulted in
some difficulties with orientation and recognizing of anatomical
structures. Histological changes observed in the samples were
identified by an experienced pathologist, blinded to the study groups,
and are presented descriptively.
Data analysis and statistics. Data were analyzed using GraphPad Prism 7.0. The D’Agostino and Pearson normality test was performed to confirm that the data conformed to a normal distribution. Body weight was analyzed by a two-way ANOVA and because of a few missing values in the dataset, a mixed model was applied. Hind leg circumferences and EVF were analyzed by a two-way repeated-measures ANOVA with Bonferroni’s multiple comparisons test. Mobility scores, stance scores, stiffness scores and ambulation impairment scores were analyzed by the non-parametric unpaired Mann-Whitney U test. P-values < 0.05 were considered statistically significant.
Results
Animal welfare assessments. All CFA-injected rats exhibited a
marked peripheral oedema, redness and acute lameness of the CFA
injected paw when observed 8 h after injection. In spite of the
pronounced inflammatory reaction, all rats where alert and interested
in their surroundings. The induced subjects did not show significantly
altered welfare assessment (WA) scores compared to the control group,
as shown in Figure 2.
|
Figure 2. Mean welfare assessment (WA) scores
of each group at different time points. The figure shows the
mean WA scores monitored daily in accordance with the score
sheet shown in Table 1. Statistical significance was determined
by a non-parametric unpaired Mann-Whitney U test and was
calculated on medians, but values are expressed as mean ±
SEM for a clear graphical presentation. No
statistically significant differences were detected between
groups. Click image to enlarge |
Prevalence of laboratory animal use for different purposes in
Algeria
The prevalence of laboratory animal use for different purposes in
Algeria (PIA report 2015) is presented in Figure 2. The percentage of
animals used in teaching activities was 41%. Most of the animals used
were small rodents (mice and rats). Only a small percentage of animals
were used for fundamental biological research (5.0%) and breeding
purposes. Animals were also used for quality control (34%) and
screening for drugs, in bioassays and for preclinical testing
including general and specific toxicity studies. The preclinical
safety and efficacy data are needed for submission to drug regulatory
authorities before permission for further studies in humans is
granted. Rabbits and horses were also frequently used for therapeutic
sera production (19%).
Body weights (BW), relative to weights on day 0, are shown in Figure
3. The CFA injected rats showed a statistically significant decrease
in body weight on day 2 (p < 0.05), with an average weight loss of
6.5% compared to the control group. From day 2 and throughout the
study, body weight increased steadily, with a growth rate that
mirrored that of the control group.
|
Figure 3. The relative body weight of each group at different time points. The asterisk (*) represents a statistically significant difference (p < 0.05) between groups throughout the entire test period, as determined by Two-Way RM ANOVA with Bonferroni’s multiple comparisons test. Values are expressed as mean ± SEM percentage change from Day 0.
Click image to enlarge |
Electronic von Frey test. Nociceptive thresholds obtained by EVF testing of the ipsilateral hind paw of CFA injected rats, compared to control animals, are shown in Figure 4 A and the contralateral hind paw compared to control animals in Figure 4 B. A statistically significant decrease (p < 0.001) in nociceptive threshold was seen in the CFA group on the ipsilateral paw during the first measurement after CFA injection (day 3) when there was the lowest measured mechanical threshold (indicating highest sensitivity). An increased mechanical threshold was found on the following days, but still with a significantly lower threshold compared to the control group on days 7 and 10 (p < 0.01). After day 14 the groups did not differ significantly. Conversely, but not statistically significant, a higher mechanical threshold was evident in the CFA group on the contralateral paw (Figure 4 B) during the entire study compared to the control group.
|
Figure 4. Electronic von Frey paw withdrawal
thresholds. A. Mechanical thresholds of the ipsilateral paw. B.
Mechanical thresholds of the contralateral paw. Effects on paw
withdrawal thresholds (g) as an expression of mechanical
hypersensitivity were determined by a Two-Way RM ANOVA with
Bonferroni’s multiple comparisons test. Asterisk represents
significant difference from the control group at *p < 0.05,
**p < 0.01 and ***p < 0.001. Data are presented as mean ±
SEM. Click image to enlarge |
Monoarthritis-specific parameters. Mobility and stance scores are shown in Figure 5 A and B. All CFA-injected rats demonstrated a statistically significant difference on the first days after CFA injection. The mobility score was significantly lower for the CFA group on day 2 (p < 0.001) and 3 (p < 0.01) compared to the control group. Similarly, the stance score, on day 2 (p < 0.001), 3 (p < 0.001), 5 (p < 0.01) and 7 (p < 0.01) was lower for the CFA group compared to the control group. The ambulation impairment (AI) score (Figure 4 B) increased significantly in the CFA group compared to the control group on the first days after CFA injection (day 3 (p < 0.001) and day 4 (p < 0.01)).
The stiffness score (Figure 5 C) significantly increased following the CFA injection and remained elevated until the end of the experiment. The highest stiffness score was seen during the first days after CFA injections (until Day 5).
The ipsilateral tibio-tarsal circumferences are presented in Figure 5
D. A significant increase (p < 0.0001) in joint circumference of
the ipsilateral joint was observed in the CFA group within 24 h of
injections and the joints remained larger compared to the control
group throughout the testing period.
|
Figure 5. Monoarthritis-specific parameters.
A. Mobility scores. B. Stance
scores. C. Ambulation impairment (AI) scores.
D. The stiffness score of the ipsilateral
tibio-tarsal joint. E. Circumference
measurements of the ipsilateral tibio-tarsal joint. Mobility
scores, stance scores, AI scores and stiffness scores were
determined by an unpaired Mann-Whitney U test and were
calculated on medians, but values are expressed as mean ± SEM in
the graphs for a clearer presentation. Differences in
circumference measurements were determined by a two-way RM ANOVA
followed by a Bonferroni post hoc test and values are expressed
as mean ± SEM. Asterisks represent a significant difference from
the control group at *p < 0.05, **p < 0.01 and ***p <
0.001. Click image to enlarge |
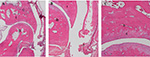
Histological examination. A representative selection of histological sections is shown in Figure 6 and 7. The selected sections of saline-injected control joints and the contralateral joints of CFA injected rats (Figure 6) showed intact cartilage and bone with normal joint space and synovial membrane. No inflammatory reaction was evident.
|
Figure 6. Hematoxylin-eosin stained section of
a normal ankle joint of a rat. Photomicrograph
A represents a normal joint, with a normal
joint space, normal intact cartilage and bone
(B) and a normal non-hyperplastic synovial
membrane (C). b, bone; c, cartilage; s,
synovial tissue; t, tibia; tb, tarsal bone. Click image to enlarge |
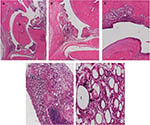
The CFA-injected tarsal joints (Figure 7) had extensive pathological
changes, both intra-articularly as well as peri-articularly. The
changes involved synovial hyperplasia, pannus formation and
infiltration of inflammatory cells (dominated by lymphocytes and
macrophages). Moreover, the sections showed bone remodelling with
increased osteoclastic activity (bone resorption) and increased
osteoblastic activity (reactive bone formation). More extensive
inflammatory changes involved periarticular tissue, which showed the
presence of empty spaces, outlined by infiltration of inflammatory
cells, surrounding lipid droplets, forming massive granulomatous
inflammatory changes.
|
Figure 7. Hematoxylin-eosin stained section of
a normal ankle joint of a rat. Photomicrograph
A represents a normal joint, with a normal
joint space, normal intact cartilage and bone
(B) and a normal non-hyperplastic synovial
membrane (C). b, bone; c, cartilage; s,
synovial tissue; t, tibia; tb, tarsal bone. Click image to enlarge |
Discussion
The present study aimed at identifying potential refinement strategies during a three-week period in a rat model of monoarthritis, induced with Freund’s adjuvant intra-articularly in the tibio-tarsal joint. The CFA-injected animals did not show significantly impaired general welfare, as assessed using the method developed by Hampshire et al. (2001) (Table 1). This suggests that the monoarthritic rat model, despite its significant inflammatory and hyperalgesic effects, is a relatively mild arthritis model in comparison to a polyarthritis model which has considerably more severe welfare implications (Pircio et al. 1975; de Castro Costaet al. 1981). However, the lack of evidence of impaired general animal welfare in the monoarthritic model is not necessarily because of the animals being unaffected by induction, but could be the result of insufficiently sensitive welfare monitoring. In other words, the welfare-monitoring scheme may simply not have been sensitive enough to detect potential suffering in the animals; this is why more extensive studies into the welfare aspects of this model are essential. Affective and motivational aspects of pain are important in this context, where persistent pain is induced. The pain can potentially be present during rest and activity and will probably have an impact on the subjects’ activity pattern. Several non-evoked measures have been suggested for investigating invoked and spontaneous pain. Among these measures are the Rat Grimace Scale (Sotocinal et al. 2011), home-cage monitoring of behavior (Urbanet al. 2011), voluntary wheel running (Coboset al. 2012), weight bearing (Tetreault et al. 2011) and gait analysis. We did not have the equipment at the time, but such methods would be of great benefit to future studies.
In general, the CFA injected rats’ welfare was more affected in
the acute phase after CFA injection than in the chronic phase. The
acute inflammatory response involved progressive tibio-tarsal joint
swelling, which gave rise to increased joint circumference, redness,
mild to moderate ambulation impairment, joint stiffness, and mobility
and stance difficulties. Weight loss was also observed in all
CFA-injected rats compared to the control animals. The two-way RM
ANOVA revealed a statistical significant decrease in mechanical
threshold in EVF in the CFA group compared to the control group; this
was lowest on day 3 but statistically significant up to day 10
indicating inflammation-evoked hypersensitivity resulting from
sensitization of the pain system. A non-significant, but possibly
biologically relevant, increase of the mechanical threshold on the
contralateral hind paw was seen in the CFA group throughout the study.
This could potentially be due to the rats’ unwillingness to
shift their weight onto the arthritic leg - a compensatory response to
the pain condition.
The acute inflammatory response was expected, but not to the extent
that was observed. Previous studies have focused on the chronic part
of the disease, without paying particular attention to the acute
phase, since the chronic phase of the disease is of interest for human
rheumatoid arthritis (Butleret al. 1992; Donaldsonet al. 1993). Data
from the present study suggest that more attention should be paid to
the acute phase of the inflammation when considering welfare
improvement and pain alleviation of the animals.
The use of analgesia to alleviate pain in inflammatory pain models is
limited, and sometimes completely neglected (Richardson and Flecknell
2005; Stokeset al. 2009). A common reason for not administering
analgesia is the concern that analgesic treatment could influence the
inflammatory response and compromise the outcome of a study. The ideal
solution would be to investigate drugs that have limited effects on
the inflammatory processes but are analgesic. This would allow for
alleviating unnecessary pain in the model. Non-steroidal
anti-inflammatory drugs would be unsuitable for use because they
directly influence the pathological development. The opioid
buprenorphine, with radically different pharmacodynamic properties, is
of special interest. Buprenorphine has been reported to have less
immunosuppressive properties than several other opioids (Sacerdote
2006) and it has been suggested to have less immunosuppressive
potential than endogenous glucocorticoids released in response to the
stress of untreated pain (Kalliokoskiet al. 2010). Future studies will
systematically investigate this matter.
The chronic phase was expected to develop 10-14 days after CFA
injections, as previously described (Butler et al. 1992; Donaldsonet
al. 1993). We, however, did not observe a pronounced chronic effect on
arthritis parameters. According to Butleret al.(1992) and Donaldson et
al. (1993), we expected a notable weight loss and a rise in
ipsilateral hind paw circumference after 10-14 days that indicates the
onset of chronic inflammation. The present study did not show further
weight loss during these days or a rise in circumference. Differences
in arthritis-related parameters between the two groups were not
obvious in the late phase of the inflammation, except for a difference
in joint stiffness. Stiffness of the joint in the acute phase is
likely to have been due to swelling from the vascular response to CFA.
Increased stiffness later in the disease process indicates chronicity,
involving synovial granulation tissue and degradation of cartilage and
bone, leading to ankyloses of the joint (Waksman 2002).
Despite sub-optimal histological sample material, monoarthritic
pathology could be confirmed in all CFA-injected rats. The sections
showed proliferative inflammatory changes, involving cell-infiltrated
synovial hyperplasia (dominated by lymphocytes and macrophages), an
inflammatory cell lining surrounding lipid droplets and pannus
formation. The lipid droplets were most likely CFA-droplets, based on
the way the immune cells surrounded the material, resembling a foreign
body reaction. Moreover, cartilage defects, bone destruction and newly
formed bone tissue were also evident. Several sections showed massive
periarticular pathological changes, suggesting strong diffusion of CFA
from the intra-articular injection site to the periphery. The right
tarsi were, however, not found to be affected, suggesting there was no
systemic spread.
The injection volume of CFA is a factor that should not be overlooked.
In the present study, we found that a small injection volume (20 µL)
of CFA injected in the tibio-tarsal joint is appropriate to model the
disease in rats in that particular joint. This is in contrast to other
studies (Besse et al. 1992; Butleret al. 1992; Donaldsonet al. 1993;
Gomes et al. 2013; Hashmiet al. 2010) where a volume of 50 µL CFA has
been used. One study (Gomes et al. 2013) has furthermore included
additional injections with CFA during the progression of the disease,
in contrast to our study where there was only one injection. This
difference in injection volumes may be explained by size or strain of
the animal, or by the experience or anatomical knowledge of the
experimenter. The development and spread of CFA-induced arthritis is
dose-dependent (Donaldsonet al. 1993), and the use of a single
injection of a small volume of CFA may have caused a less pronounced
hyperalgesic response in the present study. However, if the CFA
injection leaks out of the synovia creating inflammation in the soft
tissue surrounding the joint rather than a strict arthritic response,
using a larger volume than the one we applied might be questionable.
Thus, injecting a smaller volume could constitute a valuable
refinement of the technique, although this needs to be investigated
further.
For drug candidate efficacy testing, we consider a milder model as in
this study to be just as useful as other existing CFA-induced models
(Pearson 1956; Pearson and Wood 1959; Waksman et al. 1960; Pearson
1963; Pircio et al. 1975; de Castro Costa et al. 1981; Besse et al.
1992; Butler et al. 1992; Donaldson et al. 1993; Wu et al. 1998; Imbe
et al. 2001; Hashmi et al. 2010; Gomes et al. 2013). It should be
emphasized that we managed to induce measurable changes in EVF, model
specific parameters, and desirable histopathological changes by using
a smaller volume of CFA. In conclusion, the present study examined
refinement strategies in the adjuvant-induced monoarthritic model.
Findings in this study call for more attention to alleviating pain in
the early phase of the model progression (acute inflammation) where
animal well-being appears to be most compromised. In addition, the
study demonstrated that an injection volume of 20 µL was sufficient to
induce monoarthritis. This should be considered a refinement, since a
larger volume affects surrounding tissue that leads to unnecessary
pain and inflammation not related to the intended arthritis. However,
the effect of injection volume needs to be further investigated.
Acknowledgements
The authors wish to thank Helle Runchel Porsdal and Trine Marie Ahlman
Glahder for their technical assistance. Furthermore, thanks to Marie
Jarvad for taking care of all intra-articular injections. A special
thanks to Cathrine Juel Bundgaard, Björn Rozell and Hanne Hadberg for
histological advice and guidance. Last but not least, thanks to the
Danish 3R Center who generously supported the experimental work in
this study.
Conflict of interests
The authors declare no competing financial interest or personal
relationships that could have influ
Funding
The authors received no financial support for the research,
authorship, and/or publication of this article
References
- Besse, D., Weil-Fugazza, J., Lombard, M.C., Butler, S.H., Besson, J.M., (1992). Monoarthritis induces complex changes in mu-, delta- and kappa-opioid binding sites in the superficial dorsal horn of the rat spinal cord. European Journal of Pharmacology. 223, 123-131.
- Bolon, B., Stolina, M., King, C., Middleton, S., Gasser, J., Zack, D., Feige, U., (2011). Rodent preclinical models for developing novel antiarthritic molecules: comparative biology and preferred methods for evaluating efficacy. Journal of Biomedicine and Biotechnology. 2011, 569068. doi: 10.1155/2011/569068
- Butler, S.H., Godefroy, F., Besson, J.M., Weil-Fugazza, J., (1992). A limited arthritic model for chronic pain studies in the rat. Pain. 48, 73-81.
- Cai, X., Wong, Y.F., Zhou, H., Liu, Z.Q., Xie, Y., Jiang, Z.H., Bian, Z.X., Xu, H.X., Liu, L., (2006). Manipulation of the induction of adjuvant arthritis in Sprague-Dawley rats. Inflammation Research. 55, 368-377.
- Choudhary, N., Bhatt, L K., Prabhavalkar, K.S., (2018). Experimental animal models for rheumatoid arthritis. Immunopharmacology and Immunotoxicology. 40, 193-200.
- Cobos, E.J., Ghasemlou, N., Araldi, D., Segal, D., Duong, K., Woolf, C.J., (2012). Inflammation-induced decrease in voluntary wheel running in mice: a nonreflexive test for evaluating inflammatory pain and analgesia. Pain, 153, 876-884.
- de Castro Costa, M., de Sutter, P., Gybels, J., van Hees, J., (1981). Adjuvant-induced arthritis in rats: A possible animal model of chronic pain. Pain. 10, 173-185.
- European Union, (2010). Directive 2010/63/EU of the European Parliament and of the council of 22 September 2010 on the protection of animals used for scientific purposes. [Accessed 28 March 2020]. Available from: https://eur-lex.europa.eu/legal-content/FR/TXT/?uri=celex%3A32010L0063.
- Donaldson, L F., Seckl, J.R., McQueen, D.S., (1993). A discrete adjuvant-induced monoarthritis in the rat: effects of adjuvant dose. Journal of Neuroscience Methods. 49, 5-10.
- Festing, M.F.W., Altman, D.G., (2002). Guidelines for the design and statistical analysis of experiments using laboratory animals. ILAR journal / National Research Council, Institute of Laboratory Animal Resources. 43, 244-258.
- Festing, M.F.W., WEIGLER, B.J., (2003). Experimental design and statistical analysis. In: Hau, J., van Hoosier jr., G.L., (eds.) Handbook of laboratory animal science, vol. 1. Essential principles and practices. 2nd ed ed. Boca Raton: CRC Press.
- Gomes, R.P., Bressan, E., Silva, T.M., Gevaerd Mda, S., Tonussi, C.R., Domenech, S.C., (2013). Standardization of an experimental model suitable for studies on the effect of exercise on arthritis. Einstein (Sao Paulo). 11, 76-82.
- Hampshire, V.A., Davis, J.A., Mcnickle, C.A., Williams, L., Eskildson, H., (2001). Retrospective comparison of rat recovery weights using inhalation and injectable anaesthetics, nutritional and fluid supplementation for right unilateral neurosurgical lesioning. Laboratory Animals. 35. 223-229.
- Hashmi, J.A., Yashpal, K., Holdsworth, D.W., Henry, J.L., (2010). Sensory and vascular changes in a rat monoarthritis model: prophylactic and therapeutic effects of meloxicam. Inflammation Research. 59, 667-678.
- Imbe, H., Iwata, K., Zhou, Q.-Q., Zou, S., Dubner, R., REN, K., (2001). Orofacial Deep and Cutaneous Tissue Inflammation and Trigeminal Neuronal Activation. Implications for persistent temporomandibular pain. Cells Tissues Organs. 169, 238-247.
- Institute of Laboratory Animal Research, (2011). Guide for the Care and Use of Laboratory Animals, Washington DC, The National Academies Press.
- Kalliokoski, O., Abelson, K.S., Koch, J., Boschian, A., Thormose, S.F., Fauerby, N., Rasmussen, R.S., Johansen, F.F., Hau, J., (2010). The effect of voluntarily ingested buprenorphine on rats subjected to surgically induced global cerebral ischaemia. In Vivo. 24, 641-646.
- Kim, E.Y., Moudgil, K.D., (2009). The determinants of susceptibility/resistance to adjuvant arthritis in rats. Arthritis research & therapy. 11, 239-239.
-
Mead, R., (1988). The design of experiments, Cambridge, New
York, Cambridge University Press.
Pearson, C.M., (1956). Development of arthritis, periarthritis and periostitis in rats given adjuvants. Proceedings of the Society for Experimental Biology and Medicine, 91, 95-101. - Pearson, C.M., (1963). Experimental joint disease observations on adjuvant-induced arthritis. Journal of Chronic Disease. 16, 863-874.
- Pearson, C.M., Wood, F D., (1959). Studies of polyarthritis and other lesions induced in rats by injection of mycobacterial adjuvant. I. General clinical and pathologic characteristics and some modifying factors. Arthritis & Rheumatism. 2, 440-459.
- Pircio, A.W., Fedele, C.T., Bierwagen, M.E., (1975). New Method for Evaluation of Analgesic Activity Using Adjuvant-Induced Arthritis in Rat. European Journal of Pharmacology. 31, 207-215.
- Richardson, C.A., Flecknell, P.A., (2005). Anaesthesia and Post-operative Analgesia Following Experimental Surgery in Laboratory Rodents: Are We Making Progress? ATLA. 33, 119-127.
- Sacerdote, P., (2006). Opioids and the immune system. Palliative Medicine. 20, Suppl 1, 9-15.
- Sotocinal, S.G., Sorge, R.E., Zaloum, A., Tuttle, A.H., Martin, L.J., Wieskopf, J.S., Mapplebeck, J.C., Wei, P., Zhan, S., ZHang, S., McDougall, J.J., King, O.D., Mogil, J.S., (2011). The Rat Grimace Scale: a partially automated method for quantifying pain in the laboratory rat via facial expressions. Molecular Pain. 7, 55.
- Stokes, E.L., Flecknell, P.A., Richardson, C.A., (2009). Reported analgesic and anaesthetic administration to rodents undergoing experimental surgical procedures. Laboratory animals. 43, 149-154.
- Tag, H.M., Khaled, H.E., Ismail, H.A., El-Shenawy, N.S., (2016). Evaluation of anti-inflammatory potential of the ethanolic extract of the Saussurea lappa root (costus) on adjuvant-induced monoarthritis in rats. Journal of Basic and Clinical Physiology and Pharmacology. 27, 71-78.
- Tetreault, P., Dansereau, M.A., Dore-Savard, L., Beaudet, N., Sarret, P., (2011). Weight bearing evaluation in inflammatory, neuropathic and cancer chronic pain in freely moving rats. Physiology & Behavior. 104, 495-502.
- Urban, R., Scherrer, G., Goulding, E.H., Tecott, L.H., Basbaum, A.I., (2011). Behavioral indices of ongoing pain are largely unchanged in male mice with tissue or nerve injury-induced mechanical hypersensitivity. Pain. 152, 990-1000.
- Waksman, B.H., (2002). Immune Regulation in Adjuvant Disease and Other Arthritis Models: Relevance to Pathogenesis of Chronic Arthritis. Scandinavian Journal of Immunology. 56, 12-34.
- Waksman, B.H., Pearson, C.M., Sharp, J.T., (1960). Studies of arthritis and other lesions induced in rats by injection of mycobacterial adjuvant. II. Evidence that the disease is a disseminated immunologic response to exogenous antigen. Journal of Immunology. 85, 403-417.
- Wu, J., Lin, Q., Lu, Y., Willis, W.D., Westlund, K.N., (1998). Changes in nitric oxide synthase isoforms in the spinal cord of rat following induction of chronic arthritis. Experimental Brain Research. 118, 457-65.