Original scientific article
The anaesthetic effects of ketamine/xylazine/midazolam in C57Bl/6JRj mice
Petra Buhr,1, Stefanie Kolstrup1, Lise-Lotte
Nikolajsen1, Peter Bollen2
1
Biomedical Laboratory, University of Southern Denmark
2Department of Experimental Medicine, University of
Copenhagen
Correspondence:
Correspondence: Peter Bollen, Department of Experimental Medicine,
University of Copenhagen, Blegdamsvej 3B, DK-2200 Copenhagen N,
Denmark, E-mail:
peter.bollen@sund.ku.dk
Summary
We wished to improve the efficacy and safety of ketamine/xylazine anaesthesia in C57Bl/6JRj mice and achieve a surgical anaesthesia lasting 20-30 minutes, aiming at fewer anaesthesiarelated deaths and using the subcutaneous injection route to inflict less stress and pain on the mice. This was achieved by adding midazolam to the ketamine/xylazine anaesthetic solutions, at dosages of 193.55 mg/kg ketamine, 4.3 mg/kg xylazine and 3.76 mg/kg midazolam. This study demonstrated that subcutaneous administration of ketamine and xylazine in combination with midazolam resulted in surgical anaesthesia for at least 25 minutes. We also confirmed that a supplementary oxygen supply is necessary to maintain physiological levels of peripheral blood oxygenation in order to avoid hypoxia during ketamine/xylazine and ketamine/xylazine/ midazolam anaesthesia. As the current study was terminal, further research is needed to investigate correct dosages for recovery anaesthesia.
Introduction
A widely used protocol for ketamine/xylazine anaesthesia in mice consists of 100 mg/kg ketamine and 10 mg/kg xylazine administered by intraperitoneal (IP) injection (Fish et al. 2008; Flecknell 2023). It is our experience that this protocol does not provide satisfactory results as many of the mice need to be supplemented with anaesthetic in order to achieve surgical anaesthesia. Supplementing anaesthetic drugs always poses the risk of overdosing, resulting in profound bradycardia (Buitrago et al. 2008) and potentially death. Furthermore, IP injections are associated with risk of perforation of abdominal organs and peritoneal irritation (Levin-Arama et al. 2016) which is painful, in addition to the higher level of stress caused by handling (Henderson et al. 2020). To avoid the risks of IP injection, studies are necessary to determine if this combination of anaesthetics can be administered subcutaneously with high efficacy and safety. Using subcutaneous (SC) injection removes the risk of peritoneal irritation and perforation of abdominal organs, as well as lowering levels of pain and reducing the stress caused by handling, as the mouse can be injected with only a light scruffing with all paws still on the cage lid. This study focused on the administration route and induction of surgical anaesthesia. The experiments were terminal, so anaesthesia length and recovery were not investigated. The aim of this study was to investigate the efficacy and safety of ketamine/xylazine and ketamine/ xylazine/midazolam anaesthesia in C57Bl/6JRj mice. Improved anaesthesia contributes to reduction, as fewer anaesthesia-related deaths result in fewer animals being needed, and refinement as the SC injection route inflicts less stress and pain on the mice. The goal with ketamine/xylazine anaesthesia was to achieve a surgical anaesthesia lasting 20-30 minutes.
Materials and Methods
The study consisted of a pilot and a main experiment. The animals were housed in IVC (individually ventilated) cages (Tecniplast GM500) with aspen bedding, nesting material, a nesting box and aspen wooden block for enrichment in a barrier facility maintained at 21 ± 1oC, relative humidity of 45-65%, a light regimen of 12 hours between 6 a.m. and 6 p.m. and with ad libitum feeding (Altromin 1324, Germany). The pilot experiment included 15, 10-week-old male C57Bl/6JRj mice (Janvier Labs, France), weighing 21.5-25.4 g. The mice were housed in groups of three or four animals per cage. The animals were randomized into three treatment groups of five animals as well as order of treatment. The control group received an IP injection of 100 mg/kg ketamine (Ketaminol Vet. 50 mg/ml, MSD animal health) and 10 mg/kg xylazine (Rompun Vet. 20 mg/ml, Bayer Animal Health GmbH). The second group received a SC injection of 191.49 mg/kg ketamine and 4.26 mg/ kg xylazine. The third group received a SC injection of 193.55 mg/kg ketamine, 4.3 mg/kg xylazine and 3.76 mg/kg midazolam (Midazolam 5mg/ml Hameln Pharma GmbH). All dosages were diluted with sterile water and prepared to ensure identical injection volumes (0.1 ml/10g bodyweight) in all groups. IP injections of anaesthetics were administered by scruffing the mouse and turning it over, and SC injections by placing the mouse on the wire bar lid of the IVC cage and scruffing. The effects of inhaled oxygen on peripheral oxygen saturation and heart rate were examined in the main experiment by supplementing the animals with 100% oxygen after onset of recumbency. In the main experiment the control group was omitted as surgical anaesthesia was not achieved in the pilot experiment in any of the control animals after 10 minutes and only in 60% of these animals after 25 and 40 minutes.The main experiment included 32, 10-week-old C57Bl/6JRj mice (males and females in equal proportion), housed in groups of four animals per cage. The female mice weighed 17.7-21.0 g, and the male mice 24.0-26.8 g. The animals were randomized into the two treatments with eight animals in each of the four groups. Male and female groups were anaesthetised on two separate days, and treatment order was randomised. Females were included to determine if there was a significant difference in effect from the males. One animal from the KX female group was excluded due to incorrect dosing. The study setup is presented in Table 1.The anaesthetic was administered using the route as shown in Table 1 and the animal was placed in an IVC cage with no lid but covered with a towel to reduce the light level in the cage. When the animal was recumbent, the animal was placed on its side to verify loss of righting reflex. The animal was then moved onto a heating pad with or without oxygen supply. Ten minutes after administration of the anaesthetic, the animal was exposed to a noxious stimulus (pinching between toes on hind limb) to check for loss of pedal withdrawal reflex. If the pedal withdrawal reflex was absent, it was concluded that a surgical plane of anaesthesia had been achieved. Viscous eye-ointment (Viscotears, Bausch & Lomb Nordic ab) was also applied at this time point. If surgical anaesthesia had been achieved, heart rate (BPM) and peripheral oxygenation (SpO2) were measured using a pulse oximeter (Mousestat Junior, Kent Scientific, USA). This was repeated at intervals of 15 minutes i.e., at 10, 25 and 40 minutes after injection. At 25, 45 and 50 minutes after injection, the presence of pedal withdrawal reflex was again evaluated. The animals were euthanized after the last observation, while still anesthetised. All observations were done by a person blinded to treatment. This study was approved by the Danish Animal Experiments Inspectorate (License 2021-15-0201- 00814) and the local Animal Welfare Body.
*) One animal was excluded due to incorrect dosing.


*One of the eight injected animals did not lose consciousness (assessed using righting reflex) and was excluded from the study
**One animal regained pedal reflexes 25 minutes after injection and was euthanized
Statistics
Fisher’s exact test was performed in RStudio with adjusted p-values using the Bonferroni-Holm method. Student’s t-test and plot of residuals were performed in RStudio to detect significant differences between groups. The level of significance was set at 5%.
Results
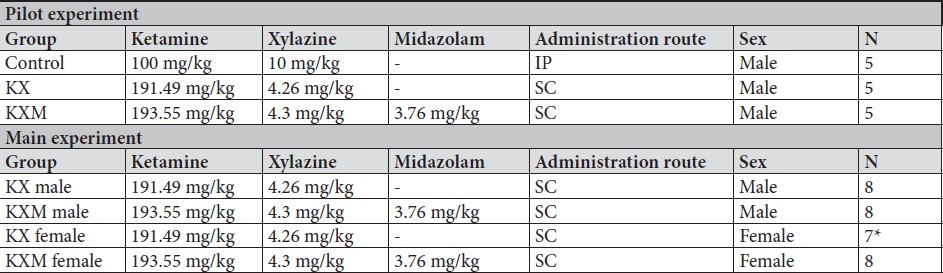
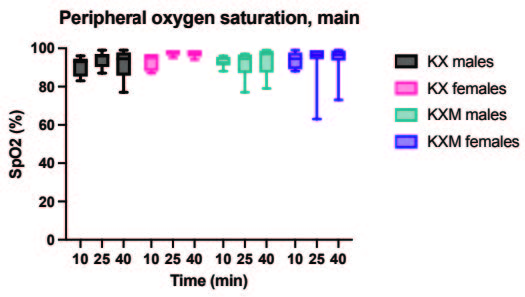
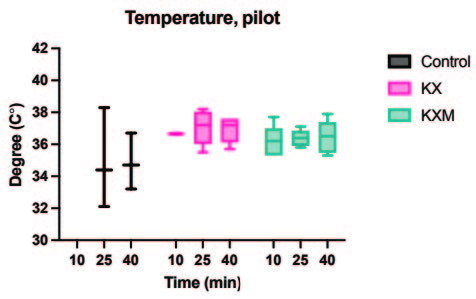
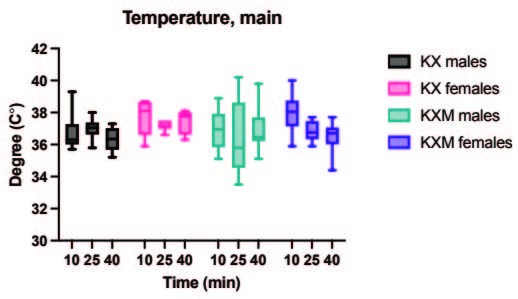
All animals in both the pilot and main experiment lost the righting reflex one to three minutes after injection, except for one female in the main experiment receiving ketamine/xylazine subcutaneously, which did not lose the righting reflex and was excluded from the study. In the pilot experiment, the solution containing midazolam was significantly more effective than the control in achieving surgical anaesthesia (lack of pedal reflex) in males after 10 minutes (p value 0.024). In the main experiment, surgical anaesthesia 10 and 25 minutes after anaesthetic dosing was achieved in all animals only when midazolam was present in the anaesthetic solution. The difference in response to the two anaesthetic solutions, however, was not statistically significant for males or females. Results are summarized in Tables 2 and 3. In the main experiment, 25 and 40 minutes after injection, the females had a significantly lower heart rate compared to males (p values 0.0347 and 0.0263). At 10 minutes the females also had a lower heart rate, although not significant. No significant differences in heart rate between treatments were found for either sex. Results are presented in Figure 1. No significant differences were seen in peripheral oxygenation in the main experiment, and values were considered normal. No significant differences were seen in heart rate and oxygen saturation in the pilot experiment, but oxygen saturation values were generally low. Results are presented in Figures 1 and 2. When comparing peripheral oxygenation in pilot and main experiments at 10, 25 and 40 minutes the peripheral oxygenation was significantly higher in the main experiment, i.e. when animals were supplemented with oxygen (p<0.05). Results are presented in Figure 2. No significant differences were found in body temperature, results are shown in Figure 3.
A. Heart rate, pilot
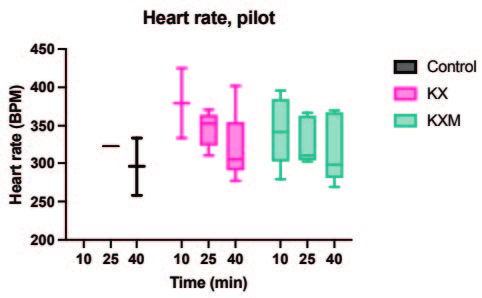
B. Heart rate, main
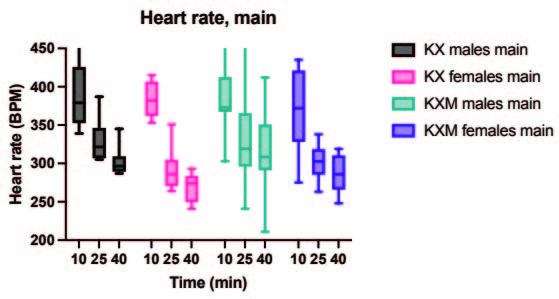
A. Peripheral oxygen saturation, pilot
B. Peripheral oxygen saturation, main
A. Body temperature, pilot
B. Body temperature, main
Discussion
Several publications have reported anaesthetic combinations of
ketamine in mice. In a systematic review of anaesthetic protocols for
urodynamic studies, ketamine/xylazine, ketamine/medetomidine, and
fentanyl/fluanisone/midazolam have been reported (Abdelkhalek et al.
2021). Ketamine and dexmedetomidine anaesthesia were among others used
in a respiratory mucociliary clearance investigation (Feldman et al.
2021), and ketamine/xylazine anaesthesia was compared to ketamine and
midazolam anaesthesia in a study of cardiac function during
echocardiography in mice (Roth et al. 2002). Acepromazine was used in
combination with ketamine and xylazine to study the effects of oxygen
supplementation on physiological parameters and depth of anaesthesia
in male and female C57BL/6 mice (Blevins et al.2021). We have not
found a recent study on the anaesthetic combination of ketamine,
xylazine and midazolam for terminal procedures, and have demonstrated
that this combination achieved surgical anaesthesia by subcutaneous
administration.
Both in the pilot and the main experiment, the solution with 193.55
mg/kg ketamine, 4.3 mg/ kg xylazine and 3.76 mg/kg midazolam used for
SC injection was effective in achieving surgical anaesthesia for at
least 25 minutes in all animals, and no significant differences were
seen in heart rate or peripheral oxygenation in comparison to the
other treatments. No animals anaesthetised with this combination
regained pedal withdrawal reflex before termination.
As surgical anaesthesia lasting 20-30 minutes was not achieved in
three out of seven females receiving the solution with 191.49 mg/kg
ketamine and 4.26 mg/kg xylazine for SC injection, this solution has
not proven effective in female C57Bl/6JRj mice. Furthermore with this
anaesthetic solution, surgical anaesthesia was only achieved in some
of the male mice in the pilot experiment 10 minutes after
administration.
A previous study reported that all mice injected intraperitoneally
with 191.25 ketamine and 4.25 mg/ kg xylazine achieved surgical
anaesthesia (Levin- Arama et al. 2016). We found that this was not the
case for both sexes in our study when subcutaneous injection was
applied, as only some of the female mice achieved surgical
anaesthesia. Even though surgical anaesthesia was not achieved within
10 minutes for some of the male mice in the pilot experiment, all had
achieved surgical anaesthesia 25 minutes after administration in our
study. This sex difference was also reported in a previous study,
showing that mouse sex, but not mode of administration, affected
whether surgical anaesthesia was achieved (Levin- Arama et al. 2016).
The addition of 3.67 mg/kg midazolam resulted in surgical anaesthesia
in both sexes after subcutaneous administration.
Anaesthetised animals not supplemented with oxygen developed severe
hypoxia. It was critical that animals anaesthetised with the
treatments included in this study received a supplementary supply of
oxygen, resulting in significantly higher levels of peripheral
oxygenation. This confirms the findings of a study into the effects of
oxygen supplementation on physiologic parameters and depth of
anaesthesia in male and female C57BL/6 mice and underlines the
importance of oxygen supplementation for all anaesthetised mice
(Blevins et al. 2021).
This study demonstrated that subcutaneous administration of ketamine
and xylazine in combination with midazolam resulted in surgical
anaesthesia for terminal procedures. However, if survival procedures
are necessary, further research into dosages of either or both
ketamine and xylazine is required. Additional investigations at our
laboratory have indicated that the ketamine dosage can be reduced to
100 mg/kg while still maintaining the efficacy and achieving a shorter
length of anaesthesia, but more research is needed to characterise
this in combination with 4.3 mg/kg xylazine and 3.76 mg/kg midazolam,
using subcutaneous administration, to ensure efficacy and safety for
both male and female mice.
All authors declare no conflict of interests.
References
- Blevins, C.E., Celeste, N.A., Marx, J.O., (2021). Effects of oxygen supplementation on injectable and inhalant anesthesia in C57BL/6 mice. Journal of the American Association for Laboratory Animal Science. 60(3), 289-297. https:// doi.org/10.30802/AALAS-JAALAS-20-000143
- Buitrago, S., Martin, T.E., Tetens-Woodring, J., Belicha- Villanueva, A., Wilding, G.E., (2008). Safety and efficacy of various combinations of injectable anesthetics in BALB/c mice.Journal of the American Association for Laboratory Animal Science. 47(1),11-17
- Feldman, K.S., Eunwon, K., Czachowski, M.J., Wu, Y., Lo, C.W., Zahid, M., (2021). Differential effect of anesthetics on mucociliary clearance in vivo in mice. Scientific Reports. 11(1), 4896. https://doi.org/10.1038/s41598-021- 84605-y
- Fish, R.E., Brown, M.J., Danneman, P.J., Karas, A.Z., ed., (2008). Anesthesia and Analgesia in Laboratory Animals. Second edition. Academic press. https://doi.org/10.1016/ B978-012373898-1.00010-4
- Flecknell, P., (2023). Laboratory Animal Anaesthesia. Fifth edition. London: Academic Press.
- Henderson, L.J., Dani, B., Serrano, E.M.N., Smulders, T.V., Roughan, J.V., (2020). Benefits of tunnel handling persist after repeated restraint, injection and anaesthesia. Scientific Reports. 10(1). https://doi.org/10.1038/s41598-020- 71476-y
- Levin-Arama, M., Abraham, L., Waner, T., Harmelin, A., Steinberg, D.M., Lahav, T., Harlev, M., (2016). Subcutaneous compared with intraperitoneal ketamine-xylazine for anesthesia of mice. Journal of the American Association for Laboratory Animal Science. 55(6), 794-800
- Roth, D.M., Swaney, J.S., Dalton, N.D., Gilpin, E.A., Ross, J.Jr, (2002). Impact of anesthesia on cardiac function during echocardiography in mice. American Journal of Physiology-Heart and Circulatory Physiology. 2826, H2134–H2140. https://doi.org/10.1152/ajpheart. 00845.2001
