Technical note
The arterial blood sampling associated with PET imaging studies can lead to post-scan complications in Göttingen minipigs
By Aage Kristian Olsen Alstrup1, Thea Pinholt Lillethorup1, Anne Marie Landau1, Pia Afzelius2
1
Department of Nuclear Medicine and PET, Aarhus University Hospital, Aarhus, Denmark.
2
Zealand University Hospital, Køge, Denmark.
Correspondence:
Aage Kristian Olsen Alstrup, Department of Nuclear Medicine & PET, Aarhus University Hospital, Palle Juul-
Jensens Boulevard 99, DK-8200 Aarhus N, Denmark.
aagealst@rm.dk
Summary
Göttingen minipigs, due to their low adult body weight, are frequently used for longitudinal imaging studies investigating disease progression or treatment effects. We recently showed that prolonged anesthesia, with intensive blood sampling and road transportation, affects internal organs in non-recovery positron emission tomography (PET) imaging studies in domestic pigs. In the current study, we examined if repeated non-invasive PET scans per se affect internal organs using computed tomography (CT). We then examined post-scan complications using clinical observations following PET imaging of Göttingen minipigs with (n=14) and without (n=4) invasive blood sampling. We report that non-invasive CT scans show no organ damage in minipigs (n=4) placed in sternal recumbence for PET scanning last-ing a few hours. Furthermore, upon reviewing medical records of other Göttingen minipigs positioned in sternal recumbence during PET scans, two cases (5 % of scans) with minor post-scan complications were found during the first two weeks after scanning. Minipigs in dorsal recumbence with the surgical placement of femoral catheters for blood sampling were more frequently (6 cases, 43 % of scans) associ-ated with minor-to-moderate post-scan complications. A reduced daily gain in body weight was also observed after minipigs were placed in dorsal recumbence with blood sampling compared to pre-scan daily weight gain. The results suggest anesthetized Göttingen minipigs are somewhat affected by short-term PET scanning procedures, and blood sampling should be reduced when possible. Post-operative care should be improved due to the higher incidence of post-scan complications in minipigs with femo-ral artery catheterization.
Introduction
Disease progression and treatment effects are frequently studied in pigs by imaging techniques. Unlike mice and rats, the brain size of pigs allows more detailed images to be acquired with positron emission tomography (PET) (Adler et al. 2022), and the scans are often performed as repeated noninvasive procedures (Alstrup and Winterdahl 2009; Alstrup and Smith 2012; Holm et al. 2016). PET imaging techniques can be used to monitor disease progression in pigs (Holm et al., 2016). For example, the progression of Parkinson’s disease (Lillethorup et al. 2018a; Lillethorup et al. 2018b; Lillethorup et al. 2018c) and the treatment effects of embryonic dopaminergic cell implantation (Dall et al. 2002) were monitored using PET scans, where it was possible to follow the dynamic brain changes in individual pigs. This type of scan provides increased biomedical insight while reducing the number of pigs used (the second R in the three Rs). Even though in vivo PET scanning studies on experimental animals are minimally invasive and, therefore, suitable for repetitive assessment of pathophysiological processes in the living body, the long-term impacts of prolonged anesthesia, body positioning, intubation, and blood sampling during scanning on an animal´s health have not been tho-roughly investigated in larger animals like pigs. Only a few papers on preclinical imaging studies provide enough information about physiological monitor-ing and follow-up care during and after the scanning. However, such information to secure animal welfare is crucial for the validity of the model and imaging data. Recently, we demonstrated in a non-recovery study that prolonged anesthesia of domestic pigs during PET scans leads to pathological changes, suggesting limitations to the duration of anesthesia for potential recovery studies (Alstrup et al. 2020). Both necropsies and computed tomography (CT) scans showed different degrees of atelectasis in the lungs (94 %) and increased fluid in the abdomen (89 %). The study was, however, conducted under extreme conditions with prolonged anesthesia (up to 18 hours), intensive blood sampling (up to 20 ml/ kg) and long road transport (1½ hours) of the pigs, and was not designed as a survival study. Furthermore, the dorsal recumbence position used during the PET scans for femoral or carotid artery exposure is unusual for a pig and can lead to lung problems after prolonged anesthesia. During the experiments, signs of pulmonary distress were monitored using CT scans. These pigs were always placed in positions that would relieve the lungs for the rest of the study (often many hours) – in most cases in sternal recumbence, but sometimes on one side with the affected lung up (Alstrup et al. 2020). For comparison, monitoring disease or treatment progression in minipigs typically includes only short-term scans (2-3 hours of anesthesia), most often without or with limited blood sampling and avoiding long road transportation. In our chronic investigations of disease progression and therapeutic strategies, we often use Göttingen minipigs (Lillethorup et al. 2018a; Lillethorup et al. 2018b; Lillethorup et al. 2018c), which are bred specifically for research purposes. However, for ethical reasons, in this study we investigated the effects of short-term PET brain scans in four Göttingen minipigs. We also reviewed the medical records and body weight changes of 19 Göttingen minipigs in other brain imaging studies.
Materials and methods
Overview of the study
This study was divided into two parts (see Figure 1). The first part presents the CT scan/re-scan data for four Göttingen minipigs (pig # 1-4) PET scanned in sternal recumbence for 2-3 hours between the CT scans (PET data not shown). The minipigs were part of an ongoing Parkinson’s disease imaging project. In the second part (without use of CT scanning), we reviewed medical records from 19 other Göttingen minipigs (pig # 5-23) used in additional brain imaging studies at our laboratory to identify potential health disorders in the weeks after PET scanning (PET data not shown). In these 19 minipigs, 14 PET scans with blood sampling and 40 PET scans without blood sampling were performed (a total of 54 scans on different dates). Some of these 19 minipigs were PET-scanned multiple times and were included in both the blood sampling and non-blood sampling groups. We reviewed medical records for the 19 minipigs acquired two weeks before and two weeks after the PET scans. We also calculated body weight changes over these approximate two-week periods. The periods were selected as close to the scan day as possible, with the pigs typically being weighed two weeks before and on the day before PET scanning, as well as two weeks after the PET scan. The minipigs were part of brain imaging projects from 2016 to 2020, and data analysis was performed blind. Experiments using these minipigs in both parts of our study were approved by the Danish Experimental Animal Inspectorate (2016−15−0201−00878 and 2019-15- 0201-01609).
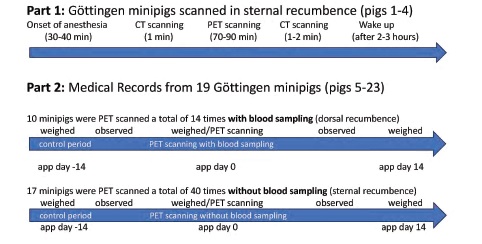
Animals
Adult (approximately 10-12 months old) female Göttingen minipigs (Ellegaard minipigs, Dalmose, Denmark; median weight 25.6 kg [quartiles: 22.2- 26.6 kg]) were single or group-housed and acclimatized for at least one month before the experiments. The environment was 12:12 hours light/dark cycle, 21-23oC, and 50-55 % humidity. The minipigs were raised under microbiologically defined conditions, fed a restricted pellet diet (SDS Diet, Witham, UK), and tap water was available ad libitum. No specific health monitoring program was followed. Only female minipigs were used in order to reduce varia-tion in dopamine imaging (Lind et al. 2005). The following procedures (clinical examinations and weighing) relate to both parts of the study but only the results from the second part of the study are reported here. Animal technicians at the pig facility clinically examined the pigs twice daily. Symptoms such as bleeding, infection, depression, loss of appetite, cough, diarrhea or lameness were recorded in the medical record for each minipig. If a minipig did not thrive, a veterinarian was immediately contacted. Furthermore, the pigs were examined by a veterinarian almost once a week. All findings, if any, were noted in the medical record. The pigs were weighed approximately on days -14, 0, and 14. At the end of the study, anesthetized minipigs were euthanized with an overdose of pentobarbitone (100 mg/kg, IV).
Scanning procedures
Before anesthesia, the pigs were fasted overnight. Premedication was induced with 6.3 mg/kg s-ketamine (Esketamin, Orifarm, Odense, Denmark) and 1.3 mg/kg midazolam (Midazolam, B. Braun, Frederiksberg, Denmark) IM. After placing a 21G Venflon (B. Braun Medical, Frederiksberg, Denmark) into an ear vein, anesthesia was induced (3.2 mg/kg s-ketamine and 1.3 mg/kg midazolam, IV). The pigs were covered with blankets. After intubation, anesthesia was maintained with inhalation of 2 % isoflurane (Baxter, Søborg, Denmark). The minipigs were mechanically ventilated for approximately 15 breaths per minute (bpm) with 1 part of oxygen and 2.2 parts of medical air (8 ml/kg). Heart rate, oxygen saturation, and body temperature were monitored during the scans. Anesthesia, ventilation or warming blankets were adjusted if the heart rate suddenly changed (> 10 % in ½ hour), oxygen saturation dropped (< 92 %) or body temperature was outside the reference interval (37-39 °C). The four CT examined minipigs (pigs #1-4) in part 1 were whole-body CT-scanned before and after the PET scanning in sternal recumbence (1½ - 2 hours between the two CT scans). In part 2, 19 minipigs (pig #5-23) were PET scanned in the absence of a whole-body CT (only attenuation CT was per-formed for correction of PET images). Forty of the PET scans were performed in sternal recumbence. For fourteen PET scans, pigs were placed in dorsal recumbence after the surgical placement of femoral artery catheters for blood sampling, (see Ettrup et al., 2010). These minipigs were in dorsal recumbence for approximately two hours (for surgery and 70-90 min of PET scanning). Approximately 80 ml of arterial blood (< 10 % of blood volume) was sampled. At the end of the scans, all catheters were removed under compression for at least ten minutes, and the skin was sutured with tight (max ½ cm) interrupted knot sutures (Dermalon 3-0). The minipigs were woken up, extubated (spon-taneous respiration), monitored for at least one hour, and treated post-surgically with 10-15 μg/kg buprenorphine (Temgesic, Indivior Europe Limited, Dublin, Ireland) IM (1 day) and 0.4 mg/kg oral mel-oxicam (Metacam, Boehringer Ingelheim, Kalund-borg, Denmark) (3 days). Minipigs without blood sampling did not receive any analgesia. Minipigs were fed when they were able to stand up again.
Examination of CT scans
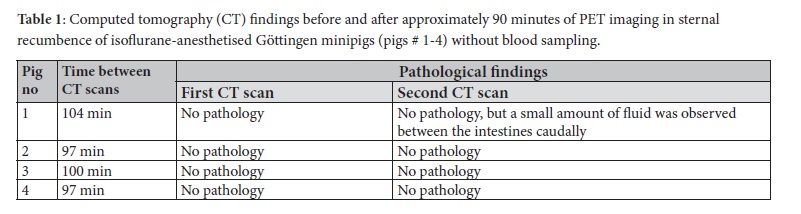
Based on the recent study by Alstrup and co-workers (Alstrup et al. 2020), any signs of atelectasis, effusions, or stasis in the lungs were recorded on wholebody CT (Siemens, Ballerup, Denmark) for pigs #1-4. The CT scans were also examined for pericardial effusion and fluid between the intestines.
Examination of medical records
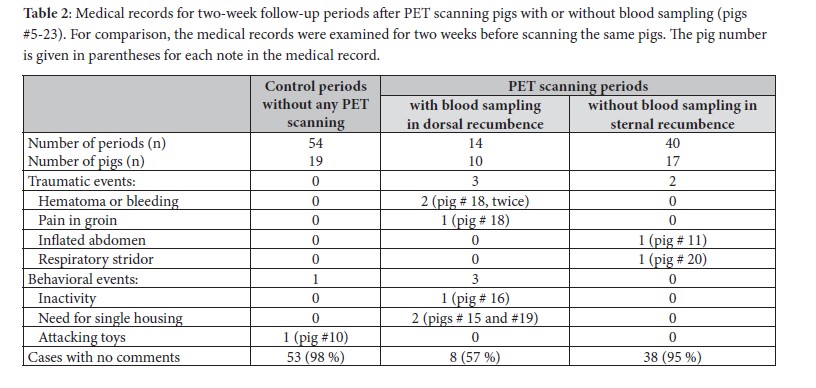
The medical records of 19 minipigs (pigs #5-23) were examined from two weeks before to two weeks after the PET scans. Any comments about symptoms and behavior were noted (information about vaccination status was excluded). Results were divided into traumatic (hematoma, bleeding, pain, abdomen inflated) and behavioral (inactive, fight, single housing) events. Furthermore, changes in body weight for the first two weeks post-scanning were compared with similar control periods for two weeks before the PET scan. To the extent possible, the control weeks were in the period immediately preceding the scans. During these periods, any written comments about the condition of the minipigs and any symptoms of illness were noted.
Statistical Analysis
To assess the distribution of our data, we employed a Quantile-Quantile (QQ) plot, which helped us determine the suitability for parametric tests. Subsequently, we used a repeated measures ANOVA to analyse the variations in weight, comparing weight changes in the post-scanning sessions to those observed during the preceding control periods. To further dissect these differences, post-hoc analyses were conducted using one-tailed t-tests with the hypothesis of a decrease in weight changes. Given our sample size constraints, we opted not to adjust for multiple comparisons in our post-hoc tests. All statistical analyses were performed using R (R Core Team, 2023).
Results
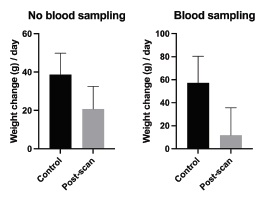
The findings of the CT scans (part 1, pigs # 1-4) are shown in Table 1. No pathological findings were detected by CT scanning following anaesthesia but before PET scanning. After PET scanning, a small amount of fluid between the intestines was found caudally in one minipig by the second CT scan. The results of the medical record examinations and body weight changes (part 2) for pigs #5-23 are shown in Table 2 and Figure 2. After 40 PET scans without blood sampling, a single pig (pig # 20) showed respiratory stridor for some minutes after extubating, and the abdomen was inflated in another pig (pig # 11) just after scanning (in total, two traumatic and no behavioural events). No negative effects were observed after 95 % of the scans. After 14 scans with blood sampling, three traumatic and three behavioural events were observed. A haematoma was observed after two different scan days in the same pig (pig # 18), and that pig also had pain in the groin after one scan. On two occasions, two other minipigs (pigs # 15 and 19) had to be temporarily single-housed to prevent domination from the cage mate. In a single case, a note was made that a minipig (pig # 16) was less active than usual, but only for one day. In 57 % of the scans, no negative effects were found. In the control period (before PET scanning), no traumatic events were observed and only a single behavioural event was noted whereat minipig (pig # 10) attacked the toys used for environmental enrichment (98 % of observations without any complications). A repeated measures ANOVA revealed a significant effect of PET scanning on weight gain (F(1, 52) = 4.078, p = 0.049). However, the interaction effect between scanning and group was not significant (F(1, 52) = 0.759, p = 0.388). In a post-hoc analysis using one-tailed t-tests, a significant difference was found between the pre- and post-scan daily weight gain in animals subjected to blood sampling (n=14) (p=0.0495). Pigs subjected to blood sampling gained an average of only 12 grams per day after PET scanning compared to 57 grams per day prior to the scan. Pigs without blood sampling showed no significant dif-ferences in body weight gain between pre- and post-scan (Figure2).
Discussion
Current preclinical research aims to provide translationally relevant scientific results and benefits from non-invasive molecular imaging techniques (PET scanning). In experimental animal studies, imaging can be used to investigate disease progression and allows the monitoring of pathophysiological characteristics of organs and tissues of interest. This study suggests that these PET imaging procedures can be done without affecting the pig’s internal organs and post-scan health, although complications can occur especially following blood sampling. In preclinical animal research, it is imperative to reduce suffering and the number of experimental animals used by following the principles of good animal ethics and the 3Rs in animal research. The strength of this study was the combination of CT scans of the minipigs (pig #1-4) and follow-up medical records after PET scanning in other pigs (pig #5-23), allowing the assessment of general health. No significant pathological findings were revealed in the small group of four minipigs undergoing CT scans in part 1. In the larger group of minipigs included in part 2, only a few minor to moderate complications were observed in the first two weeks after the end of PET scanning (43% of scans with blood sampling compared with 5 percent of scans without blood sampling versus 2 percent of observations in the control period). Blood sampling during scans is sometimes necessary to obtain information about blood and plasma radioactivity and the presence of metabolites, which are used for kinetic modelling of the PET data. In the group of minipigs that underwent femoral arterial blood sampling, one minipig had negative findings. These findings were likely associated with the dorsal recumbence positioning, the femoral surgery, and the arterial blood sampling. Based on our experi-ence with humans, we expect that a small amount of declivity stasis (venous congestions and light oedema in the lungs) can occur after 30-40 min. but will subside when the minipig wakes up and is no longer in the same unnatural position. The observed bleeding and hematomas were limited and did not give rise to further treatments or euthanasia of the minipigs. A few minipigs were inactive or had to be temporarily single-housed. The inactivity could have been due to surgery or weakening after blood collection. However, the blood sample volume was below the maximum of 10 percent of the blood volume allowed within a two to three week period. The complications after blood sampling suggest that although the severity of findings was minor to moderate, repeated blood sampling should be limited whenever possible and that monitoring the pigs thoroughly during the days after the operation is also vital. Additionally, the average values for daily weight gains were lower after scanning with blood sampling, and were statistically different from the small positive median weight gain during the control (pre-scan) periods. It was impossible to determine whether this reduced weight gain persisted past two weeks. The minipigs that did not undergo blood sampling had no significant changes in body weight gains. An advantage of this study is that it could be based on existing study data, so no new animal experimentation was necessary, consistent with the concept of re-using existing data in animal science. There were also limitations associated with this study that merit discussion. It was only possible to CT scan four minipigs (part 1) for financial reasons. Furthermore, only 14 minipig PET scans were included in the blood sampling group in part 2. Hoof trimming, simple blood sampling, and similar routine procedures were performed in some cases which may have impacted the data. However, it is unlikely that these procedures have had significant effects on the results, as they were limited compared to the influence of anesthesia, surgery and scanning. Clinical observations were not standardized, and the animal caretakers may have had different thresholds for reporting observations that were only sometimes specifically defined. However, the same animal caretakers have cared for all the animals. In some cases, single housing was necessary to avoid fighting among stall mates. Furthermore, the minipigs were part of other ongoing investigations. They may, therefore, have been more vulnerable to developing post-scan complications.
Conclusion
The pig is an essential large animal model for neurological and cardiometabolic PET research. The insight into the well-being and stress factors that might influence this animal model during PET scans and, thus, interpretation of the imaging data, is essential. We examined whether short-term PET scans and post-scan blood sampling affect the pig’s internal organs. Our results do not indicate that the pigs are significantly affected by short-term scanning in sternal recumbence. However, some traumatic and behavioral complications were observed after arterial blood sampling in dorsal recumbence. A decrease in body weight gain was observed after blood sampling. Despite this, it seems reasonable to carry out repeated short-term PET/CT experiments in minipigs to study disease mechanisms or therapies; researchers should however minimize additional complications associated with, for example, blood sampling.
Acknowledgements
We thank Dr. Michael Winterdahl for support with the statistical analysis.
Conflicts of Interest
The authors declare that they have no conflict of interests.
References
- Adler, S.S., Seidel, J., Choyke, P.L., (2022): Advances in preclinical PET. Seminars in Nuclear Medicine. 52(3), 382- 402.
- Alstrup, A.K.O., Smith, D.F., (2012). PET neuroimaging in pigs. Scandinavian Journal of Laboratory Animal Science. 39(1), 25-45.
- Alstrup, A.K.O., Winterdahl, M., (2009). Imaging techniques in large animals. Scandinavian Journal of Laboratory Animal Science. 36(1), 55-66.
- Alstrup, A.K.O., Afzelius, P., Jensen, S.B., Leifsson, P.S., Wegener, K.M., Nielsen, O.L., (2020). Effects of long-term anesthesia, blood sampling, transportation, and infection status on hearts and brains in pigs inoculated with Staphylococcus aureus and used for imaging studies. Journal of the American Association for Laboratory Animal Science. 59, 1-11.
- Holm, I.E., Alstrup, A.K.O., Luo, Y., (2016). Genetically modified pig models for neurodegenerative disorders. Journal of Pathology. 238(2), 267-287.
- Dall, A.M., Danielsen, E.H., Sørensen, J.C., Andersen, F., Møller, A., Zimmer, J., Gjedde, A.H., Cumming, P., (2002). Quantitative [18F]fluorodopa/PET and histology of fetal mesencephalic dopaminergic grafts to the striatum of MPTP-poisoned minipigs. Cell Transplantation. 11, 733- 746.
- Ettrup, K.S., Glud, A.N., Orlowski, D., Fitting, L.M., Meier, K., Soerensen, J.C., Bjarkam, C.R., Alstrup, A.K.O., (2011). Basic surgical techniques in the Göttingen minipig: intubation, bladder catheterization, femoral vessel catheterization, and transcardial perfusion. Journal of Visualized Experiments. 52, e2652, 1-5.
- Lillethorup, T.P., Glud, A.N., Alstrup, A.K.O., Noer, O., Nielsen, E.H.T., Schacht, A.C., Landeck, N., Kirik, D., Orlowski, D., Sørensen, J.C.H., Doudet, D.J., Landau, A.M., (2018a). Longitudinal monoaminergic PET imaging of chronic proteasome inhibition in minipigs. Scientific Reports. 8, 15715.
- Lillethorup, T.P., Glud, A.N., Alstrup, A.K.O., Mikkelsen, T.W., Nielsen, E.H., Zaer, H., Doudet, D.J., Brooks, D.J., Sørensen, J.C.H., Orlowski, D., Landau, A.M., (2018b). Nigrostriatal proteasome inhibition impairs dopamine neurotransmission and motor function in minipigs. Experimental Neurology. 303, 142-152.
- Lillethorup, T.P., Glud, Landeck, N., Alstrup, A.K.O., Jakobsen, S., Vang, K., Doudet, D.J., Brooks, D.J., Kirik, D., Hinz, R., Sørensen, J.C., Landau, A.M., (2018c). In vivo quantification of glial activation in minipigs overexpressing human alphasynuclein. Synapse. 72, e22060
- Lind, N.M., Olsen, A.K., Moustgaard, A., Jensen, S.B., Jakobsen, S., Hansen, A.K., Arnfred, S.M., Hemmingsen, R.P., Gjedde, A., Cumming, P., (2005). Mapping the amphetamine-evoked dopamine release in the brain of the Göttingen minipigs. Brain Research Bulletin. 65, 1, 1-9.
