Development of concurrent infection of notoedric mange in rabbits infected with Trypanosoma evansi
by L. D. Singla1*, Neena Singla2 & V. R.
Parshad2
1Department of Veterinary Parasitology, Guru Angad Dev
Veterinary and Animal Sciences University, Ludhiana-141004, India
2Department of Zoology, Punjab Agricultural University,
Ludhiana-141004, India.
 Correspondence: Dr. Lachhman Das Singla, Professor -cum-Head,
Correspondence: Dr. Lachhman Das Singla, Professor -cum-Head,
Department of Veterinary Parasitology, College of Veterinary Science,
GADVASDU, Ludhiana-141004, Punjab, India.
Phone: +91 161 2414029,
Fax: +91 161 2400822,
E-mail: ldsingla@gmail.com
Summary
This report describes observations on the development of cutaneous lesions in rabbits experimentally infected with Trypanosoma evansi. Skinscrapings revealed the presence of different developmental stages of Notoedres cati var. cuniculi and the animals were successfully treated by combined anti-Trypanosoma (diminazene aceturate @ 3.5 mg.kg-1body weight) and acaricid treatments (doramectin @ 400mg.kg-1body weight). T. evansi infection probably made the rabbits more prone to infection of notoedric mange and doramectin proved to be effective, practical and well tolerated means of treatment.
Introduction
Trypanosoma evansi, a mechanically transmitted blood protozoan parasite causing trypanosomosis (‘surra’) is one of the major obstacles to livestock production and health (Gill, 1991). Besides causing disease, the trypanosomes are held responsible for producing a state of severe immunosuppression which renders the host more susceptible to secondary infections and poor response to bacterial and viral vaccines (Holmes, 1980). Mange is one of the serious debilitating and common skin disorder reported in rabbits throughout the world (Davies et al, 1991). Transmission among animals is by direct contact and humans are infected rarely (Chakrabarti, 1986). Common hosts of notoedric mange include cats, rats, squirrels, rabbits and bats (Mullen & Oconnor, 2002). Several drugs have been tried against notoedric mange in rabbits with varied efficacy (Singla et al, 1996; Chhabra et al, 1989; Singari et al, 2001; Aulakh et al, 2003). Doramectin, a long acting endectocide against ectoparasites with a wide spectrum of activity (Logan et al, 1996; Jagannath & Yathiraj, 1999), has been successfully used as a single dose treatment against mange mite in sheep (Bates et al, 1995), pigs (Arends et al, 1999) and camel (Singh et al, 2001). During our studies on the potential of T. evansi as biocide of rodent pest species viz. Bandicota bengalensis and Rattus rattus, T. evansi strain was maintained in rabbits which acquired notoedric mange. The objective of the present study is to place on record the development of naturally acquired notoedric mange (due to Notoedres cati var. cuniculi) in rabbits experimentally infected with T. evansi along with its successful treatment.
Materials and methods
Animals
Sexually mature healthy laboratory bred rabbits between 1-2 years of
age (n=8) of both sexes (four each), with mean body weight of 2.43 ±
0.18 kg, were kept in single rabbit metallic cages in a well
ventilated room for the maintenance a source of
T. evansi. The floor, walls and ceiling of the room were
smooth and free from cracks and crevices. Rabbits were fed with
standard animal diet procured from M/s Ashirwad Industries
(Chandigarh), India. During the study period, the temperature and
humidity ranged between 22-280C and 40-60%, respectively. The rabbits
had access to natural day light conditions. Animal cages and rooms
were cleaned daily. Animals were treated humanely as per the
guidelines approved by Institutional Animal Ethics Committee, Punjab
Agricultural University (PAU), Ludhiana, India.
Infection and clinical signs
Prior to the use of rabbits for maintenance of T. evansi, all
the rabbits (both treated and untreated) were checked for the presence
of ecto- and endoparasites through blood, fecal and skin scrapings
examination. All the rabbits were found negative both for ecto and
endoparasites. For maintaining T. evansi, five rabbits (two
males and three females) with mean body weight of 2.18 ± 0.21 kg were
inoculated intraperitoneally with 1 ml of the purified trypanosome
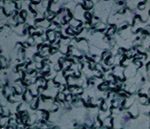
(Figure 1) stock containing 1 x 106 T. evansi. The rabbits
developed signs of disease with paroxysms and intermission. The
infected rabbits were found to have intense itching, discomfort and
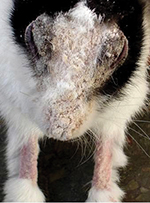
loss of fur with patchy exudation from the skin due to dermatitis.
This was followed by dry yellowish white crust formation. All the
cases presented lesions on the frontal area of head including nose,
lips, and eye lids. Three rabbits also showed lesions on pinna and
legs (Figure 2). Heavy crust on eyes interfered with vision. The
animals were also found depressed and emaciated. These clinical signs
and characteristics of lesions were suggestive of mange. Other rabbits
(two males and one female with mean body weight of 2.50 ± 0.32 kg) not
infected with T. evansi remained normal and healthy.
Diagnosis
Definitive diagnosis was confirmed by microscopic examination of skin
scrapings of all the five rabbits. Scrapings of the crusty skin of the
affected rabbits were collected, treated with 10% KOH solution and
incubated at 37oC for 8 hours followed by centrifugation at 800g for 5
minutes. The supernatant was discarded and the sediment was carefully
mixed with saturated glucose solution. Finally, after 10 minutes the
upper layer was collected and examined under a stereoscopic microscope
to determine the presence of mites. Specific identification of the
mites was performed following the description given by Soulsby (1982)
and Muller et al (1983). Biopsy of skin preserved in 10%
neutral buffered formalin was conducted for recording
histopathological changes. Skin scrapings of healthy, uninfected
rabbits were also examined for the presence of mites. These rabbits
did not have clinical signs of mange.
Treatment
All the infected rabbits were treated with doramectin @ 400 mg.Kg-1
body weight subcutaneously. The mean dose of doramectin calculated for
5 infected rabbits was 872 ± 83.33µg per rabbit with a range of 600 to
1200µg per rabbit. Because of the difficulties associated with topical
treatment of rabbits and in administering the small drug volume
required i.e. less than 100µl per rabbit, a decision was made to treat
all the infected rabbits with a dose of 0.1ml of 1% (10mg.ml-1) injectable solution of doramectin (Dectomax, marketed by
Pfizer Limited, Pfizer center, Mumbai, India) subcutaneously for
notoedric mange. Simultaneously, the uninfected rabbits were also
injected with 0.1ml distilled water subcutaneously. Injection of
diminazene aceturate (Berenil, Hoechst India Limited) @ 3.5mg.Kg-1
body weight was given along with antihistamine chlorphenaramine
maleate to treat trypanosomosis.
After the treatment, rabbits were observed daily for general and local adverse reactions, increase or decrease in scratching, restlessness and lesions, reduction in crust, appearance of the hair coat and improvement in general behaviour. Blood smear examination and mouse inoculation tests (Figure 3) were conducted daily for three days and subsequently at fortnightly intervals to diagnose trypanosomosis. Skin scrapings of treated rabbits were obtained after the doramectin treatment on day 3, 7, 10, 15 and then at fortnightly intervals. Follow up evaluations of all the rabbits continued for up to 3 months after doramectin and diminazene aceturate treatment.
Results
Pre-treatment
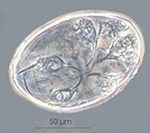
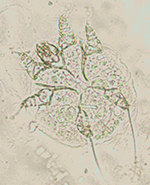
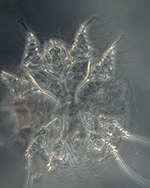
Microscopic examination of skin scrapings revealed abundant mites at
various developmental stages i.e. egg, egg containing larva, larva,
nymph and adult (Figures 4-7) indicating active infection. The number
of mites/cm2 including all the stages varied from 40 to 120 per skin
scraping with a mean of 78 ±13.28. The mites were identified as
Notoedres cati var. cuniculi on the basis of long
and unjointed pedicels with bell shaped suckers on tarsi (Figures
6-7), location of anus at dorsum (Figure 7) and lacking of dorsal
spines which differentiates Notoedres spp. from
Sarcoptes spp. as per the description given in Muller
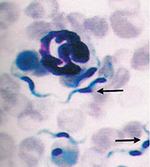
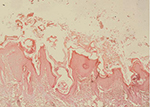
et al (1983). Skin biopsy revealed changes in the epidermis
varying from inflammation (with infiltration of eosinophils,
lymphocytes, fibroblasts and neutrophils), occasional areas of
hemorrhages, edema, hyperplasia and hyperkeratosis. The mites did not
burrow beyond the epidermal region. The cut sections of mites were
present in the superficial layers of the epidermis (Figure 8). No mite
was observed in the skin scrapings of healthy, uninfected rabbits.
Post-treatment
No adverse signs (nervous signs, depression, lethargy, diarrhea,
blindness, abnormal behaviour, inflammation or pain at the site of
injection) were observed after the administration of doramectin and
diminazene aceturate in any of the treated cases. The treatment
resulted in progressively decreased frequency of itching. The animals
stopped scratching completely within 5 days of the treatment. On day
7, skin scrapings were found negative for all the developmental stages
of N. cati var. cuniculi except adult mites. Rabbits
were negative for T. evansi from day 2 onwards as revealedby
blood smear examination and mouse inoculation test. Hair started
growing and the animals improved visibly in condition. Complete cure
was observed within 15 days of treatment as evidenced by complete lack
of visually detectable lesions, absence of mites in skin scrapings,
growth of hair and improvement in general condition of the animals
(Figure 9). Up to 3 months after treatment, animals did not show any
clinical signs and were parasitologically (blood and skin scraping
examination) negative for the presence of mites and trypanosomes.
Discussion
The rabbits used for the maintenance of strain of T. evansi may be sub-clinically infected with notoerdric mange. T. evansi infection may have made them prone to develop the clinical form of disease which was suspected from skin lesions and was easily diagnosed by skin scraping examination parasitologically. Notoedric mange has also been reported previously in other rabbits kept with the breeder (PAU, Ludhiana) and other parts of the city which were successfully treated with acaricides (Singla et al, 1996; Aulakh et al, 2003). Immunosuppression caused by T. evansi infections has been observed in cattle (Ikeme et al, 1984), Buffalo (Singla et al, 2001), goat (Sharma et al, 2000), pig (Holland et al, 2003) and guinea pig (Sarmah et al, 1997) from time to time by various workers. Flaring up of T. evansi infection due to immunosuppression caused by mange infection leading to mortality in pigs has also been observed (Gill et al, 1987).
The results of present studies that N. cati var. cuniculi mites did not burrow beyond superficial layers of epidermis in rabbits are confirmatory of the previous reports (Singla et al, 1996, Rajeswari et al, 1995) in rabbits. Pence and colleagues (Pence et al, 1982), however, reported occurrence of several notoedric mites in stratum corneum and a few in stratum germinativum of the bobcat.
In comparative trials of doramectin and ivermectin (Arends et al, 1999; Clymer et al, 1997), persistent efficacy of doramectin was found to be much longer on the basis of prevention of infestation and mite recovery. This may be due to its longer plasma half life than ivermectin and high concentration achieved in the skin to kill the mite present in the keratin layer of the skin (Goudie et al., 1993; Jones et al, 1993; Reinmeyer & Courteny,, 2001). The long plasma life of doramectin is attributable to its oily formulation and its non-polar cyclohexyl group located at carbon 25 of the ivermectin ring (Wicks et al, 1993). Based on these factors when doramectin @ 400mg.kg-1 body weight was injected to rabbits as a single dose, it was successful for bringing clinical and parasitological recovery from notoedric mange in rabbits infected with T. evansi. These results are similar to those reported by Delucchi & Castro (2000) against notoedric mange in cats. Voyvoda et al (2005) found the drug very effective against sarcoptic mange in angora rabbits.
One of the interesting observations during present studies was that all the rabbits which were not infected with T. evansi were healthy and clinically and parasitologically negative for notoedric mange during and after the experimentation. Perusal of available literature indicates that it appears to be the first report of its kind due to the development of notoedric infection concurrent to trypanosomosis in rabbits with 100 percent recovery following a single dose of doramectin.
References
-
Arends JJ, TL Skogerboe & LK Ritzhanpt. Persistent
efficacy of doramectin and ivermectin against experimental
infestations of Sarcoptes scabei var. suis in
swine. Vet. Parasitol., 1999, 82, 71-79.
- Aulakh GS, J Singh, LD Singla & N Singla. Pathology and therapy of natural notoedric acariosis in rabbits. J. Vet. Parasitol., 2003, 17, 127-29.
-
Bates PG, BA Groves, SA Courteney & GC Coles. Control
of sheep scab (Psoroptes ovis) on artificially infested
sheep with a single injection of doramectin. Vet. Rec., 1995,
137, 491-21.
-
Chakrabarti A. Human notoedric scabies from contact with
cats infested with Notoedres cati. Int. J. Dermatol., 1986,
25, 646-48.
-
Chhabra C, DC
Nauriyal, SS Khahra & SD Bhadwaj. Efficacy of
moxidectin in notodectic mange in rabbits. Indian J. Vet. Med.,
1999, 19, 109.
-
Clymer BC, TH Jones & ME McKenzie. Evaluation of
therapeutic and protective efficacy of doramectin against
Psoroptes scabiei in cattle. Vet. Parasitol.,
1997, 72, 79-89.
-
Davies PR, MJ Moore & AM Pointon. Seasonability of
sarcoptic mange in pigs in South Australia. Australian Vet.
J., 1991, 68, 390-92.
-
Delucchi L & E Castro. Use of doramectin for the
treatment of notoedric mange in five cats. J. Am.Vet. Med. Assoc.,
2000, 216, 215-16.
-
Gill BS. Trypanosomes and trypanosomiases of Indian
livestock, 1st revised edition, Indian Council of Agricultural
Research, New Delhi, 1991.
-
Gill BS, J Singh, JS Gill & Kwatra MS.
Trypanosoma evansi infection in pigs in India. Vet. Rec.,
1987, 120, 92.
-
Goudie AC, NA Evans, KA Gration, BF Bishop , SP Gibson, KS Holdom, B Kaye, SR Wicks, D Lewis, AJ Weatherley &
et al. Doramectin-a potent novel endectocide. Vet.
Parasitol., 1993, 49, 5-15.
-
Holland WG, TT Do, NT Huong, TV Dung, NG Thanh, J Vercruysse
& BM Goddeeris. The effect of T. evansi infection on pig performance and
vaccination against classical swine fever. Vet. Parasitol., 2003,
111, 115-23.
-
Holmes PH. Vaccination against trypanosomes. In: Vaccines
against parasites, AER Taylor & R Muller (eds.), 1st
edn. Blackwell and Scientific Publications, Oxford, UK, 1980.
-
Ikeme MM, AA Saharee, MZ Saad & R Koran. Effect of
Experimental Trypanosoma evansi infection on cattle and
subsequent response to haemorrhagic septicaemia vaccine. Tropical
Veterinaria, 1984, 2, 61-67.
-
Jagannath MS & S Yathiraj. Clinical evaluation of
doramectin in the treatment of ectoparasites of canines. Indian Vet.
J., 1999, 76, 333-34.
-
Jones RM, NB Logan, AJ Weatherley, AS Little & CD
Smothers.
Activity of doramectin against nematode endoparasites of cattle.
Vet. Parasitol.,1993, 49, 27-37.
-
Logan NB, AJ Weatherley & RM Jones. Activity of
doramectin against nematode and arthropod parasites of swine. Vet.
Parasitol., 1996, 66, 87-94.
-
Mullen GR & Oconnor BM. Mites (Acari). In: Medical and
Veterinary Entomology, G Mullen & L Durden (eds.), Academic
Press, UK, 2002, 449-510.
-
Muller GH, FW Kirk & DW Scot. Small animal dermatology,
3rd edn., W B Saunders, Co, Philadephia, 1983, 331-52.
-
Pence DB, FD Matthew & LA Windberg. Notoedric mange in
the bobcat, Felis rufus from South Texas. J.Wildlife
Diseases, 1982, 18, 47-50.
-
Rajeswari YB, K Sattyanaran, MS Jagannath, U Venkatesh & YB
Sumathi. Studies on histopathological changes of rabbit mange.
Parasitology and Applied Animal Biology, 1995, 4, 97-98.
-
Reinmeyer CR & CH Courteny. Antinematodal drugs. In:
Veterinary Pharmacology and Therapeutics, HR Adams (ed.), State
University Press, Ames, Lowa, 2001, 947-79.
-
Sarmah PC, Y Bhattacharyulu & VB Yoshi. Effect of
Trypanosoma evansi infection on the antibody response in
guinea pigs vaccinated with haemorrhagic septicaemia oil adjuvant
vaccine. J. Vet. Parasitol., 1997, 11, 161-164.
-
Sharma DK, PPS Chauhan & RD Agrawal. Interaction
between Trypanosoma evansi and Haemonchus contortus
infection in goats. Proc. XI National Cong. Vet. Parasitol., 2000,
75-76.
-
Singari NA, VR Kasaralikar, B Shobhamani & DC Choudhuri. Notoedric mange in rabbits and its treatment with doramectin. J.
Vet. Parasitol., 2001, 15, 77-78.
-
Singh V, RR Momin & HR Parsani. Therapeutic efficacy of
doramectin against sarcoptic mange in camels. J. Vet. Parasitol.,
2001, 15, 75-76.
-
Singla LD, PD Juyal & PP Gupta. Therapeutic trial of
ivermectin against Notoedres cati var.
cuniculi infection in rabbits. Parasite, 1996,
3, 87-89.
-
Singla LD, PD Juyal, NS Sharma & A Singh. Responses to
haemorrhagic septicaemia vaccination in
Trypanosoma evansi infected buffalo-calves. Proc. 10th Int.
Conf. AITVM, Copenhagen, Denmark, 20-23 August, 2001, 481 pp.
-
Soulsby EJL. Helminths, Arthropoda and Protozoa of domestic
animals, 7th edn, Balliere Tindall, London, 1982.
-
Voyvoda H, B Ulutas, H Eren, T Karagenc & G Bayramli.
Use of doramectin for treatment of sarcoptic mange in five Angora
rabbits. Vet. Dermatol., 2005, 6, 285-88.
- Wicks SR, B Kaye, AJ Weatherly, D Lewis, E Davison, SP Gibson & DG Smith. Effect of formulation on the pharmacokinetic and efficacy of doramectin. Vet. Parasitol., 1993, 49, 17-26.